Sourdough “Biga” Fermentation Improves the Digestibility of Pizza Pinsa Romana: An Investigation through a Simulated Static In Vitro Model
Abstract
:1. Introduction
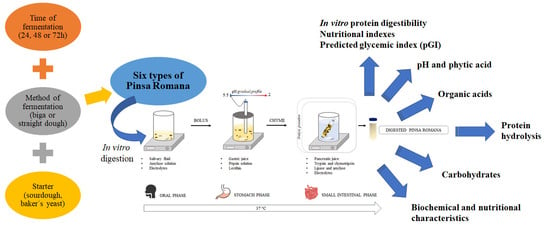
2. Materials and Methods
2.1. Raw Materials
2.2. Sourdough Preparation
2.3. Biga and PRs Making
2.4. Physical and Microbiological Characterization of Biga and SD
2.5. In Vitro Static Digestion Procedure
2.6. Biochemical and Nutritional Characterization of the PRs
2.6.1. Nutritional Label
2.6.2. pH, Total Treatable Acidity (TTA) and Phytic Acid
2.6.3. In Vitro Protein Digestibility (IVPD), Nutritional Indexes and Predicted Glycemic Index (pGI)
2.7. Biochemical and Nutritional Analysis before and after Small Intestinal In Vitro Digestion of PRs
2.7.1. Organic Acids, Sugars, and Dietary Fibers
2.7.2. Total and Free Individual Amino Acids, Total Peptides and Determination of Peptide Profiles by Reversed-Phase Fast Performance Liquid Chromatography (RP-FPLC)
2.8. Statistical Analysis
3. Results
3.1. Characterization of Sourdough and Biga
3.2. Nutritional Label of PRs
3.3. Biochemical and Nutritional Characteristics of PRs
3.3.1. pH, TTA and Phytic Acid
3.3.2. IVPD, pGI and Nutritional Indexes
3.4. Organic Acids before and after Small Intestinal In Vitro Digestion of PRs
3.5. Carbohydrates before and after Small Intestinal In Vitro Digestion of PRs
3.6. Protein Hydrolysis before and after Small Intestinal In Vitro Digestion of PRs
3.6.1. Total Peptides and RP-FPLC Peptide Profiles
3.6.2. Release of FAA
3.7. Principal Component Analysis (PCA) on PR Common Variables before and after Small Intestinal In Vitro Digestion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cauvain, S.P.; Clark, R.H. Baking Technology and Nutrition; Wiley: Hoboken, NJ, USA, 2019; ISBN 9781119387152. [Google Scholar]
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough Bread: A Contemporary Cereal Fermented Product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; La Storia, A.; Gobbetti, M.; Di Cagno, R. Microbial Ecology Dynamics during Rye and Wheat Sourdough Preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoriello, T.; Mellara, F.; Bartoli, L.; Galli, V.; Amoriello, M. Innovation in Pinsa Romana Production: A Response Surface Methodology Approach. Chem. Eng. Trans. 2021, 87, 619–624. [Google Scholar] [CrossRef]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Sodi, C.; Granchi, L. Influence of Different Leavening Agents on Technological and Nutritional Characteristics of Whole Grain Breads Obtained from Ancient and Modern Flour Varieties. Eur. Food Res. Technol. 2021, 247, 1701–1710. [Google Scholar] [CrossRef]
- Balestra, F.; Pinnavaia, G.G.; Romani, S. Evaluation of the Effects of Different Fermentation Methods on Dough Characteristics. J. Texture Stud. 2015, 46, 262–271. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in Sourdough Fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Costantini, A.; Da, A.; Nikoloudaki, O.; Montemurro, M.; Di, R.; Genot, B.; Gobbetti, M.; Giuseppe, C. How Cereal Flours, Starters, Enzymes, and Process Parameters Affect the in Vitro Digestibility of Sourdough Bread. Food Res. Int. 2022, 159, 111614. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, e00494-21. [Google Scholar] [CrossRef]
- González-Alvarado, J.M.; Jiménez-Moreno, E.; Lázaro, R.; Mateos, G.G. Effect of Type of Cereal, Heat Processing of the Cereal, and Inclusion of Fiber in the Diet on Productive Performance and Digestive Traits of Broilers. Poult. Sci. 2007, 86, 1705–1715. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef]
- Capuano, E.; Janssen, A.E.M. Food Matrix and Macronutrient Digestion. Annu. Rev. Food Sci. Technol. 2021, 12, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Mamone, G.; Nitride, C.; Addeo, F.; Ferranti, P. Protein Digestomics: Integrated Platforms to Study Food-Protein Digestion and Derived Functional and Active Peptides. TrAC Trends Anal. Chem. 2013, 53, 178–186. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meletis, C.D.; Barker, J.E. Therapeutic Uses of Amino Acids. Altern. Complement. Ther. 2005, 11, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.M.; Madalena, D.A.; Pinheiro, A.C.; Vicente, A.A. Rice in Vitro Digestion: Application of INFOGEST Harmonized Protocol for Glycemic Index Determination and Starch Morphological Study. J. Food Sci. Technol. 2020, 4, 445–473. [Google Scholar] [CrossRef] [Green Version]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch Structure Influences Its Digestibility: A Review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in Vitro and in Vivo Data on Food Digestion. What Can We Predict with Static in Vitro Digestion Models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and Challenges in Modeling Human Gastric and Small Intestinal Digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Carafa, I.; Fava, F.; Tuohy, K.M.; Nikoloudaki, O.; Gobbetti, M.; Cagno, R. Di Sourdough Performances of the Golden Cereal Tritordeum: Dynamics of Microbial Ecology, Biochemical and Nutritional Features. Int. J. Food Microbiol. 2022, 374, 109725. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Rigby, N. InfoGest Consensus Method. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; ISBN 9783319161044. [Google Scholar]
- Koirala, P.; Costantini, A.; Maina, H.N.; Rizzello, C.G.; Verni, M.; De Beni, V.; Polo, A.; Katina, K.; Cagno, R.D.; Coda, R. Fermented Brewers’ Spent Grain Containing Dextran and Oligosaccharides as Ingredient for Composite Wheat Bread and Its Impact on Gut Metabolome In Vitro. Fermentation 2022, 8, 487. [Google Scholar] [CrossRef]
- Baldini, M.; Fabietti, F.; Giammarioli, S.; Onori, R.; Orefice, L.; Stacchini, A. Metodi Di Analisi Utilizzati per Il Controllo Chimico Degli Alimenti; Rapporti ISTISAN 96/34; Istituto Superiore Della Sanità: Rome, Italy, 1996. [Google Scholar]
- AACC. Approved Methods of Analysis, 11th ed.; AOAC: St. Paul, MN, USA, 2010. [Google Scholar]
- Akeson, W.R.; Stahmann, M.A. A Pepsin Pancreatin Digest Index of Protein Quality Evaluation. J. Nutr. 1964, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of Fungal Proteases and Selected Sourdough Lactic Acid Bacteria for Making Wheat Bread with an Intermediate Content of Gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, e254. [Google Scholar] [CrossRef]
- Church, A.C. Vasopressin Potentiates the Stimulation of Cyclic AMP Accumulation by Norepinephrine. Peptides 1983, 4, 261–263. [Google Scholar] [CrossRef]
- Doi, E.; Shibata, D.; Matoba, T. Modified Colorimetric Ninhydrin Methods for Peptidase Assay. Anal. Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; AOAC Press: Rockville, MA, USA, 2005. [Google Scholar]
- Pintér-Szakács, M.; Molnár-Perl, I. Determination of Tryptophan in Unhydrolyzed Food and Feedstuffs by the Acid Ninhydrin Method. J. Agric. Food Chem. 1990, 38, 720–726. [Google Scholar] [CrossRef]
- OSER, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins. In Protein and Amino Acid Nutrition; Academic Press: Cambridge, MA, USA, 1959; pp. 281–295. [Google Scholar] [CrossRef]
- Ihekoronye, A.I. A Rapid in Vitro Enzymic and Chromatographic Predictive Model for the in Vivo Rat-based Protein Efficiency Ratio of Mixed Food Proteins. Food/Nahrung 1988, 32, 789–793. [Google Scholar] [CrossRef]
- CRISAN, E.V.; SANDS, A. Nutritional Value. In The Biology and Cultivation of Edible Mushrooms; Academic Press: New York, NY, USA, 1978. [Google Scholar] [CrossRef]
- Block, R.J.; Mitchell, H.H. The Correlation of the Amino Acid Composition of Proteins with Their Nutritive Value. Nutr. Abstr. Rev. 1946, 16, 249–278. [Google Scholar]
- Capriles, V.D.; Arêas, J.A.G. Effects of Prebiotic Inulin-Type Fructans on Structure, Quality, Sensory Acceptance and Glycemic Response of Gluten-Free Breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A Starch Hydrolysis Procedure to Estimate Glycemic Index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Weiss, W.; Vogelmeier, C.; Görg, A. Electrophoretic Characterization of Wheat Grain Allergens from Different Cultivars Involved in Bakers’ Asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, G.; Conterno, L.; Gerbi, V. Determination of Organic Acids, Sugars, Diacetyl, and Acetoin in Cheese by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2001, 49, 2722–2726. [Google Scholar] [CrossRef]
- AOAC (International AOAC International). Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Rockville, MA, USA, 2012. [Google Scholar]
- De Pasquale, I.; Verni, M.; Verardo, V.; Gómez-Caravaca, A.M.; Rizzello, C.G. Nutritional and Functional Advantages of the Use of Fermented Black Chickpea Flour for Semolina-Pasta Fortification. Foods 2021, 10, 182. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; di Palo, D.M.; Lorusso, M.P.; de Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough Fermented Breads Are More Digestible than Those Started with Baker’s Yeast Alone: An in Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef] [Green Version]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough Bread: Starch Digestibility and Postprandial Glycemic Response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Serra, A.; Conte, G.; Flamini, G.; Angelini, L.G. Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake. Appl. Sci. 2020, 10, 5235. [Google Scholar] [CrossRef]
- De Angelis, M.; Damiano, N.; Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Sourdough Fermentation as a Tool for the Manufacture of Low-Glycemic Index White Wheat Bread Enriched in Dietary Fibre. Eur. Food Res. Technol. 2009, 229, 593–601. [Google Scholar] [CrossRef]
- Maioli, M.; Pes, G.M.; Sanna, M.; Cherchi, S.; Dettori, M.; Manca, E.; Farris, G.A. Sourdough-Leavened Bread Improves Postprandial Glucose and Insulin Plasma Levels in Subjects with Impaired Glucose Tolerance. Acta Diabetol. 2008, 45, 91–96. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. Influence of Sourdough on in Vitro Starch Digestibility and Predicted Glycemic Indices of Gluten-Free Breads. Food Funct. 2014, 58, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.; Björck, I. Delayed Gastric Emptying Rate May Explain Improved Glycaemia in Healthy Subjects to a Starchy Meal with Added Vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östman, E.M.; Nilsson, M.; Liljeberg Elmståhl, H.G.M.; Molin, G.; Björck, I.M.E. On the Effect of Lactic Acid on Blood Glucose and Insulin Responses to Cereal Products: Mechanistic Studies in Healthy Subjects and In Vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation 2023, 9, 90. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and Bacterial Conversions during Sourdough Fermentation. Food Microbiol. 2014, 205, 31–36. [Google Scholar] [CrossRef]
- Saha, B.C.; Racine, F.M. Biotechnological Production of Mannitol and Its Applications. Appl. Microbiol. Biotechnol. 2011, 87, 553–560. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Calasso, M.; Campanella, D.; De Angelis, M.; Gobbetti, M. Use of Sourdough Fermentation and Mixture of Wheat, Chickpea, Lentil and Bean Flours for Enhancing the Nutritional, Texture and Sensory Characteristics of White Bread. Int. J. Food Microbiol. 2014, 180, 78–87. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food Processing for the Improvement of Plant Proteins Digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef]
- Sozer, N.; Melama, L.; Silbir, S.; Rizzello, C.G.; Flander, L.; Poutanen, K. Lactic Acid Fermentation as a Pre-Treatment Process for Faba Bean Flour and Its Effect on Textural, Structural and Nutritional Properties of Protein-Enriched Gluten-Free Faba Bean Breads. Foods 2019, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of Natural and Lactobacillus Plantarum Fermentation on In-Vitro Protein and Starch Digestibilities of Sorghum Flour. Food Biosci. 2013, 2, 46–52. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv. Nutr. 2018, 9, 849–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of Angiotensin I-Converting Enzyme (ACE)-Inhibitory Peptides and γ-Aminobutyric Acid (GABA) during Sourdough Fermentation by Selected Lactic Acid Bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the Protein Quality of Wheat Bread through Faba Bean Sourdough Addition. LWT 2017, 82, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of Sourdough Fermentation and Pseudo-Cereals and Leguminous Flours for the Making of a Functional Bread Enriched of γ-Aminobutyric Acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef]
- Gallego-Lobillo, P.; Ferreira-Lazarte, A.; Hernández-Hernández, O.; Villamiel, M. In Vitro Digestion of Polysaccharides: InfoGest Protocol and Use of Small Intestinal Extract from Rat. Food Res. Int. 2021, 140, 110054. [Google Scholar] [CrossRef]
- Tamura, M.; Singh, J.; Kaur, L.; Ogawa, Y. Impact of the Degree of Cooking on Starch Digestibility of Rice—An in Vitro Study. Food Chem. 2016, 191, 98–104. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Alfieri, F.; Gallo, V.; Miralles, B.; Masi, P.; Romano, A.; Ferranti, P.; Recio, I. Compared Digestibility of Plant Protein Isolates by Using the INFOGEST Digestion Protocol. Food Res. Int. 2020, 137, 109708. [Google Scholar] [CrossRef]
- Qi, X.; Al-Ghazzewi, F.H.; Tester, R.F. Dietary Fiber, Gastric Emptying, and Carbohydrate Digestion: A Mini-Review. Starch/Staerke 2018, 70, 1700346. [Google Scholar] [CrossRef]
- Maekawa, M.; Ushida, K.; Hoshi, S.; Kashima, N.; Ajisaka, K.; Yajima, T. Butyrate and Propionate Production from D-Mannitol in the Large Intestine of Pig and Rat. Microb. Ecol. Health Dis. 2005, 17, 169–176. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-Enabled Wellness Foods: A Fresh Perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-promoting Properties of Its Derived-products. Fermentation 2021, 7, 246. [Google Scholar] [CrossRef]




| Ingredients (%, w/w) | PR_24 | PR_48 | PR_48(SD) | PR_48+SD | PR_72 | PR_RT | |
|---|---|---|---|---|---|---|---|
| Pinsa Romana dough | |||||||
| Wheat flour | - | - | - | - | - | 50 | |
| Rice flour | - | - | - | - | - | 4 | |
| Soy flour | - | - | - | - | - | 1.5 | |
| Biga * | 88 | 88 | 90 | 73 | 88 | - | |
| Wheat flour | 51 | 51 | 49 | 42 | 51 | - | |
| Rice flour | 4 | 4 | 4.5 | 4 | 4 | - | |
| Soy flour | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | - | |
| Water | 31 | 31 | 29 | 25 | 31 | - | |
| Baker’s yeast | <1 | <1 | <1 | <1 | <1 | - | |
| Sourdough | - | - | 5 | - | - | - | |
| Water | 10 | 10 | 8 | 5 | 10 | 42 | |
| Baker’s yeast | - | - | <1 | <1 | - | <1 | |
| Sourdough | - | - | - | 19 | - | - | |
| Olive oil | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |
| Salt | 1 | 1 | 1 | 1 | 1 | 1 | |
| PR_24 | PR_48 | PR_48(SD) | PR_48+SD | PR_72 | PR_RT | |
|---|---|---|---|---|---|---|
| Moisture (%) | 36.5 | 34.3 | 31.5 | 32.5 | 33.4 | 35.5 |
| Total Fat (g/100 g) | 5.7 | 5.6 | 5.4 | 5.9 | 5.5 | 5.8 |
| Total Carbohydrate (g/100 g) | 75.8 | 76.0 | 77.4 | 76.8 | 76.9 | 76.8 |
| Ash (g/100 g) | 2.5 | 2.7 | 2.4 | 2.5 | 2.4 | 2.5 |
| Protein (g/100 g) | 16.0 | 15.8 | 14.8 | 14.8 | 15.2 | 14.9 |
| Energy value (Kcal/100 g) | 413 | 406 | 411 | 417 | 412 | 411 |
| pH | TTA | Phytic Acid | IVPD | pGI | |
|---|---|---|---|---|---|
| PR_24 | 5.61 ± 0.01 a | 2.9 ± 0.1 d | 0.12 ± 0.02 a | 62.9 ± 0.4 a | 68.8 ± 0.3 a |
| PR_48 | 5.48 ± 0.02 b | 3.3 ± 0.1 c | 0.11 ± 0.02 a | 63.0 ± 0.8 a | 67.8 ± 0.8 a |
| PR_48(SD) | 4.27 ± 0.02 d | 8.4 ± 0.3 a | 0.09 ± 0.01 a | 60.3 ± 0.4 a | 63.9 ± 0.1 b |
| PR_48+SD | 4.90 ± 0.02 c | 5.1 ± 0.1 b | 0.09 ± 0.02 a | 60.6 ± 1.1 a | 68.0 ± 1.1 a |
| PR_72 | 5.41 ± 0.04 b | 3.1 ± 0.1 cd | 0.11 ± 0.01 a | 62.7 ± 1.9 a | 68.5 ± 0.4 a |
| PR_RT | 5.60 ± 0.03 a | 2.3 ± 0.1 e | 0.12 ± 0.02 a | 62.2 ± 1.4 a | 71.3 ± 1.8 a |
| PR_24 | PR_48 | PR_48(SD) | PR_48+SD | PR_72 | PR_RT | |
|---|---|---|---|---|---|---|
| EAA index | 68.97 ± 3.73 a | 68.99 ± 4.13 a | 72.61 ± 3.50 a | 71.55 ± 4.44 a | 75.26 ± 4.87 a | 75.85 ± 5.70 a |
| BV index | 63.48 ± 4.11 a | 63.50 ± 3.82 a | 67.44 ± 4.22 a | 66.29 ± 4.75 a | 70.33 ± 4.26 a | 70.98 ± 3.98 a |
| PER | 22.36 ± 1.71 bc | 24.69 ± 2.11 b | 30.33 ± 1.54 a | 23.32 ± 1.05 bc | 24.37 ± 1.61 b | 19.19 ± 1.29 c |
| NI | 2.91 ± 0.13 b | 2.86 ± 0.12 b | 3.87 ± 0.27 a | 3.03 ± 0.12 b | 3.60 ± 0.17 a | 2.77 ± 0.19 b |
| Sequence of Limiting EAA | Leu | Lys | Thr | Lys | His | Met |
| His | Trp | Lys | Thr | Lys | Leu | |
| Trp | Leu | His | His | Thr | His |
| Lactic Acid | Acetic Acid | Glucose | Fructose | Mannitol | Maltose | Peptides | RS | Dietary Fibers | |
|---|---|---|---|---|---|---|---|---|---|
| Pinsa Romana | |||||||||
| PR_24 | 2.9 ± 0.1 c | <LOQ | 11.0 ± 0.1 d | 6.7 ± 0.1 b | <LOQ | 46.7 ± 0.1 c | 9.3 ± 0.1 d | 1.1 ± 0.1 a | 14.4 ± 0.6 a |
| PR_48 | 2.5 ± 0.6 c | <LOQ | 10.0 ± 0.1 d | 5.8 ± 0.1 b | <LOQ | 35.4 ± 0.1 e | 9.5 ± 0.1 d | 0.8 ± 0.2 ab | 15.4 ± 1.7 a |
| PR_48(SD) | 102.1 ±0.1 a | 27.0 ± 0.1 a | 32.7 ± 0.1 a | 2.3 ± 0.1 d | 41.8 ± 0.1 a | 60.6 ± 0.1 b | 12.7 ± 0.2 a | 0.9 ± 0.1 ab | 14.8 ± 1.3 a |
| PR_48+SD | 35.7 ± 0.1 b | 14.7 ± 0.3 b | 17.6 ± 0.1 c | 4.5 ± 0.1 c | 6.2 ± 0.1 b | 43.9 ± 0.1 d | 12.0 ± 0.1 b | 0.7 ± 0.1 b | 14.8 ± 0.8 a |
| PR_72 | 2.3 ± 0.1 c | 1.2 ± 0.3 c | 21.1 ± 0.1 b | 14.0 ± 0.1 a | <LOQ | 61.0 ± 0.1 b | 11.4 ± 0.1 c | 0.8 ± 0.1 ab | 13.7 ± 0.7 a |
| PR_RT | 2.6 ± 0.2 c | <LOQ | 3.1 ± 0.3 e | 6.9 ± 0.1 b | <LOQ | 74.7 ± 0.1 a | 9.1 ± 0.2 d | 1.0 ± 0.1 ab | 14.2 ± 1.7 a |
| Digested (D) Pinsa Romana | |||||||||
| D-PR_24 | <LOQ | <LOQ | 144.4 ± 12.1 ab | 13.3 ± 0.1 b | 0.9 ± 0.6 c | 699.6 ± 23.7 a | 145.2 ± 7.2 c | 0.7 ± 0.1 a | 15.3 ± 1.7 a |
| D-PR_48 | <LOQ | <LOQ | 137.3 ± 8.2 b | <LOQ | 0.1 ± 0.1 c | 722.9 ± 35.2 a | 159.4 ± 6.9 bc | 0.8 ± 0.1 a | 14.0 ± 0.2 a |
| D-PR_48(SD) | 42.0 ± 3.0 a | <LOQ | 188.5 ± 3.3 a | <LOQ | 21.0 ± 2.1 a | 741.2 ± 15.2 a | 201.2 ± 8.1 a | 0.9 ± 0.1 a | 13.4 ± 0.9 a |
| D-PR_48+SD | 18.7 ± 0.6 b | <LOQ | 149.7 ± 15.0 ab | <LOQ | 4.4 ± 2.3 b | 728.1 ± 55.1 a | 183.1 ± 1.5 ab | 0.9 ± 0.1 a | 16.6 ± 0.1 a |
| D-PR_72 | <LOQ | <LOQ | 146.1 ± 4.5 ab | 15.2 ± 1.1 a | <LOQ | 709.9 ± 45.3 a | 165.8 ± 1.3 bc | 0.9 ± 0.1 a | 16.0 ± 0.7 a |
| D-PR_RT | <LOQ | <LOQ | 126.9 ± 22.7 b | 12.9 ± 0.2 b | <LOQ | 665.8 ± 41.4 a | 146.9 ± 2.3 c | 0.9 ± 0.1 a | 16.6 ± 1.1 a |
| CH3CN < 19% | 19% < CH3CN < 41% | CH3CN > 41% | Total AUC | |
|---|---|---|---|---|
| Pinsa Romana | ||||
| PR_24 | 12.9 | 87.1 | 0 | 2511.3 |
| PR_48 | 17.5 | 82.6 | 0 | 2659.0 |
| PR_48(SD) | 19.1 | 80.9 | 0 | 3693.0 |
| PR_48+SD | 5.8 | 94.2 | 0 | 3273.8 |
| PR_72 | 22.6 | 77.4 | 0 | 2713.8 |
| PR_RT | 18.1 | 81.8 | 0 | 2196.9 |
| Digested (D) Pinsa Romana | ||||
| D-PR_24 | 67.5 | 17.5 | 15.1 | 3307.5 |
| D-PR_48 | 63.2 | 20.3 | 16.5 | 3594.8 |
| D-PR_48(SD) | 58.8 | 28.9 | 12.4 | 3478.6 |
| D-PR_48+SD | 66.5 | 18.4 | 15.1 | 3185.7 |
| D-PR_72 | 66.0 | 21.5 | 12.5 | 3518.8 |
| D-PR_RT | 66.5 | 18.4 | 15.1 | 3611.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, A.; Verni, M.; Mastrolonardo, F.; Rizzello, C.G.; Di Cagno, R.; Gobbetti, M.; Breedveld, M.; Bruggink, S.; Lefever, K.; Polo, A. Sourdough “Biga” Fermentation Improves the Digestibility of Pizza Pinsa Romana: An Investigation through a Simulated Static In Vitro Model. Nutrients 2023, 15, 2958. https://doi.org/10.3390/nu15132958
Costantini A, Verni M, Mastrolonardo F, Rizzello CG, Di Cagno R, Gobbetti M, Breedveld M, Bruggink S, Lefever K, Polo A. Sourdough “Biga” Fermentation Improves the Digestibility of Pizza Pinsa Romana: An Investigation through a Simulated Static In Vitro Model. Nutrients. 2023; 15(13):2958. https://doi.org/10.3390/nu15132958
Chicago/Turabian StyleCostantini, Alice, Michela Verni, Federica Mastrolonardo, Carlo Giuseppe Rizzello, Raffaella Di Cagno, Marco Gobbetti, Mario Breedveld, Suzan Bruggink, Kristof Lefever, and Andrea Polo. 2023. "Sourdough “Biga” Fermentation Improves the Digestibility of Pizza Pinsa Romana: An Investigation through a Simulated Static In Vitro Model" Nutrients 15, no. 13: 2958. https://doi.org/10.3390/nu15132958
APA StyleCostantini, A., Verni, M., Mastrolonardo, F., Rizzello, C. G., Di Cagno, R., Gobbetti, M., Breedveld, M., Bruggink, S., Lefever, K., & Polo, A. (2023). Sourdough “Biga” Fermentation Improves the Digestibility of Pizza Pinsa Romana: An Investigation through a Simulated Static In Vitro Model. Nutrients, 15(13), 2958. https://doi.org/10.3390/nu15132958