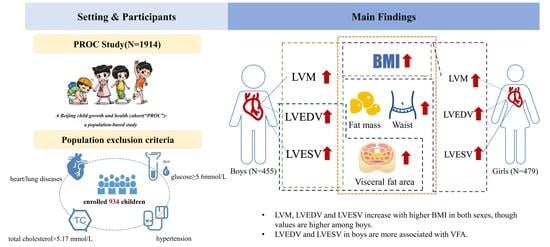
Sex-Specific Differences in Left Ventricular Mass and Volumes with Body Mass Index among Children Aged 6 to 8: A Cross-Sectional Study in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Inclusion Criteria
2.3. Laboratory Assays and Anthropometric Measurements
2.4. Echocardiography Measurement
2.5. Statistical Analysis
3. Results
3.1. Blood Pressure and Left Ventricular (LV) Function
3.2. Sex Differences in LV Hypertrophy and Volume Size Considering Obesity
3.2.1. LV Hypertrophy and Volume Size in Boys
3.2.2. LV Hypertrophy and Volume Size in Girls
3.3. Comparison of Sex-Specific Hypertrophy and Volume Size in Obesity
3.3.1. Left Ventricular Mass (LVM)
3.3.2. LV End-Diastolic Volume (LVEDV)
3.3.3. LV End-Systolic Volume (LVESV)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šileikienė, R.; Adamonytė, K.; Ziutelienė, A.; Ramanauskienė, E.; Vaškelytė, J.J. Atrial and Ventricular Structural and Functional Alterations in Obese Children. Medicina 2021, 57, 562. [Google Scholar] [CrossRef] [PubMed]
- Mintjens, S.; Menting, M.D.; Daams, J.G.; van Poppel, M.N.M.; Roseboom, T.J.; Gemke, R.J.B.J. Cardiorespiratory Fitness in Childhood and Adolescence Affects Future Cardiovascular Risk Factors: A Systematic Review of Longitudinal Studies. Sports Med. 2018, 48, 2577–2605. [Google Scholar] [CrossRef] [Green Version]
- Bakkum, M.J.; Danad, I.; Romijn, M.A.J.; Stuijfzand, W.J.A.; Leonora, R.M.; Tulevski, I.I.; Somsen, G.A.; Lammertsma, A.A.; Van Kuijk, C.; van Rossum, A.C.; et al. The Impact of Obesity on the Relationship between Epicardial Adipose Tissue, Left Ventricular Mass and Coronary Microvascular Function. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1562–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, S.; Reis, J.P.; Venkatesh, B.A.; Gidding, S.S.; Armstrong, A.C.; Jacobs, D.R., Jr.; Sidney, S.; Wu, C.O.; Cook, N.L.; Lewis, C.E.; et al. Race-Ethnic and Sex Differences in Left Ventricular Structure and Function: The Coronary Artery Risk Development in Young Adults (Cardia) Study. J. Am. Heart Assoc. 2015, 4, e001264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Konja, D.; Wang, L.; Wang, Y. Sex Differences in Adiposity and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 9338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, S.; Bazzano, L.; He, J.; Whelton, P.; Chen, W. Trajectories of Childhood Blood Pressure and Adult Left Ventricular Hypertrophy: The Bogalusa Heart Study. Hypertension 2018, 72, 93–101. [Google Scholar] [CrossRef]
- Crowley, D.I.; Khoury, P.R.; Urbina, E.M.; Ippisch, H.M.; Kimball, T.R. Cardiovascular Impact of the Pediatric Obesity Epidemic: Higher Left Ventricular Mass Is Related to Higher Body Mass Index. J. Pediatr. 2011, 158, 709–714.e1. [Google Scholar] [CrossRef]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left Ventricular Hypertrophy and Hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Rodicio, M.M.; de Miguel, V.D.; Jiménez, M.G.; Guldrís, S.C.; Franco, M.M.L.; Gestal, A.E.; Couce, M.L.; Trabazo, M.R.L. Early Cardiac Abnormalities in Obese Children and Their Relationship with Adiposity. Nutrition 2018, 46, 83–89. [Google Scholar] [CrossRef]
- Lieb, W.; Gona, P.; Larson, M.G.; Aragam, J.; Zile, M.R.; Cheng, S.; Benjamin, E.J.; Vasan, R.S. The Natural History of Left Ventricular Geometry in the Community: Clinical Correlates and Prognostic Significance of Change in Lv Geometric Pattern. JACC. Cardiovasc. Imaging 2014, 7, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.L.; Maahs, D.M.; Garvey, K.C.; Hood, K.K.; Laffel, L.M.; Weinzimer, S.A.; Wolfsdorf, J.I.; Schatz, D. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, 2026–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National, Heart Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension Guidelines for the Management of High Blood Pressure in Children and Adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [Green Version]
- Devereux, R.B. Detection of Left Ventricular Hypertrophy by M-Mode Echocardiography. Anatomic Validation, Standardization, and Comparison to Other Methods. Hypertension 1987, 9, II19–II26. [Google Scholar] [CrossRef] [Green Version]
- Teichholz, L.E.; Kreulen, T.; Herman, M.V.; Gorlin, R. Problems in Echocardiographic Volume Determinations: Echocardiographic-Angiographic Correlations in the Presence of Absence of Asynergy. Am. J. Cardiol. 1976, 37, 7–11. [Google Scholar] [CrossRef]
- Bartkowiak, J.; Spitzer, E.; Kurmann, R.; Zürcher, F.; Krähenmann, P.; Garcia-Ruiz, V.; Mercado, J.; Ryffel, C.; Losdat, S.; Llerena, N.; et al. The Impact of Obesity on Left Ventricular Hypertrophy and Diastolic Dysfunction in Children and Adolescents. Sci. Rep. 2021, 11, 13022. [Google Scholar] [CrossRef]
- Lee, J.W.; Hong, Y.M.; Kim, H.S. Identification of Cardiovascular Risk Factors in Obese Adolescents with Metabolic Syndrome. Front. Pediatr. 2021, 9, 745805. [Google Scholar] [CrossRef]
- Lind, L.; Michaelsson, K.; Söderberg, S.; Larsson, A.; Johansson, L.; Kullberg, J.; Ahlström, H.; Sundström, J. On the Association between Body Fat and Left Ventricular Mass. J. Hypertens. 2019, 37, 1699–1704. [Google Scholar] [CrossRef]
- Saner, C.; Harcourt, B.E.; Pandey, A.; Ellul, S.; McCallum, Z.; Kao, K.T.; Twindyakirana, C.; Pons, A.; Alexander, E.J.; Saffery, R.; et al. Sex and Puberty-Related Differences in Metabolomic Profiles Associated with Adiposity Measures in Youth with Obesity. Metabolomics 2019, 15, 75. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Dhutia, H.; D’Silva, A.; Malhotra, A.; Steriotis, A.; Millar, L.; Prakash, K.; Narain, R.; Papadakis, M.; Sharma, R.; et al. Effect of Sex and Sporting Discipline on Lv adaptation to Exercise. JACC Cardiovasc. Imaging 2017, 10, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Lin, Y.; Liu, Y.; Liu, F.; Liu, X.; Dong, W.; Cai, W.; Chen, H.; Xiao, M.; et al. Anthropometric Parameters of Obesity Can Be Alternative Biomarkers for the Potential Cardiac Dysfunction in Obese Children. Front. Cardiovasc. Med. 2022, 9, 850071. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, J.S.; Hernesniemi, J.A.; Ruohonen, S.; Hutri-Kähönen, N.; Kähönen, M.; Jokinen, E.; Tossavainen, P.; Kallio, M.; Laitinen, T.; Lehtimäki, T.; et al. Influence of Early-Life Body Mass Index and Systolic Blood Pressure on Left Ventricle in Adulthood—The Cardiovascular Risk in Young Finns Study. Ann. Med. 2021, 53, 160–168. [Google Scholar] [CrossRef]
- Rider, O.J.; Lewandowski, A.; Nethononda, R.; Petersen, S.E.; Francis, J.M.; Pitcher, A.; Holloway, C.J.; Dass, S.; Banerjee, R.; Byrne, J.P.; et al. Gender-Specific Differences in Left Ventricular Remodelling in Obesity: Insights from Cardiovascular Magnetic Resonance Imaging. Eur. Heart J. 2013, 34, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Hardy, R.; Ghosh, A.K.; Deanfield, J.; Kuh, D.; Hughes, A.D. Birthweight, Childhood Growth and Left Ventricular Structure at Age 60-64 Years in a British Birth Cohort Study. Int. J. Epidemiol. 2016, 45, 1091–1102. [Google Scholar] [CrossRef] [Green Version]
- Sawada, N.; Nakanishi, K.; Daimon, M.; Yoshida, Y.; Ishiwata, J.; Hirokawa, M.; Nakao, T.; Morita, H.; Di Tullio, M.R.; Homma, S.; et al. Influence of Visceral Adiposity Accumulation on Adverse Left and Right Ventricular Mechanics in the Community. Eur. J. Prev. Cardiol. 2020, 27, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Winters-van Eekelen, E.; Van der Velde, J.H.; Boone, S.C.; Westgate, K.; Brage, S.; Lamb, H.J.; Rosendaal, F.R.; De Mutsert, R. Objectively Measured Physical Activity and Body Fatness: Associations with Total Body Fat, Visceral Fat, and Liver Fat. Med. Sci. Sports Exerc. 2021, 53, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Dahl-Petersen, I.K.; Brage, S.; Bjerregaard, P.; Tolstrup, J.S.; Jørgensen, M.E. Physical Activity and Abdominal Fat Distribution in Greenland. Med. Sci. Sports Exerc. 2017, 49, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.; Godino, J.; Nichols, J.F.; Wing, D.; Hill, L.; Patrick, K. Associations between Physical Activity and Bmi, Body Fatness, and Visceral Adiposity in Overweight or Obese Latino and Non-Latino Adults. Int. J. Obes. 2017, 41, 873–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.H.; Kim, H.K.; Bae, S.J.; Lee, M.J.; Hwang, J.Y.; Choe, J.; Jung, C.H.; Lee, W.J.; Park, J.Y. Gender Differences of Visceral Fat Area for Predicting Incident Type 2 Diabetes in Koreans. Diabetes Res. Clin. Pract. 2018, 146, 93–100. [Google Scholar] [CrossRef]
- Ballin, M.; Lundberg, E.; Sörlén, N.; Nordström, P.; Hult, A.; Nordström, A. Effects of Interval Training on Visceral Adipose Tissue in Centrally Obese 70-Year-Old Individuals: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2019, 67, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variable | Normal Weight | Overweight | Obese | |||
|---|---|---|---|---|---|---|
| Boys (n = 357) | Girls (n = 389) | Boys (n = 51) | Girls (n = 57) | Boys (n = 47) | Girls (n = 33) | |
| Age (years) | 7.1 ± 0.3 | 7.1 ± 0.3 | 7.1 ± 0.3 | 7.1 ± 0.3 | 7.2 ± 0.3 | 7.1 ± 0.3 |
| BMI (kg/m2) | 15.4 ± 1.1 *,†,‡ | 15.0 ± 1.2 §,|| | 19.1 ± 0.8 ** | 18.8 ± 0.8 †† | 23.0 ± 1.9 | 22.3 ± 1.5 |
| SBP (mmHg) | 100.6 ± 7.2 *,‡ | 98.2 ± 7.1 § | 100.7 ± 7.9 ** | 101.1 ± 8.2 | 105.5 ± 7.0 ‡‡ | 99.4 ± 7.4 |
| DBP (mmHg) | 55.2 ± 5.7 | 55.1 ± 5.4 § | 56.6 ± 5.6 | 57.2 ± 5.6 | 55.8 ± 6.1 | 55.8 ± 6.8 |
| Waist circumference (cm) | 54.1 ± 3.7 *,†,‡ | 52.4 ± 3.6 §,|| | 64.7 ± 4.2 ¶,** | 62.9 ± 4.3 †† | 74.8 ± 6.3 ‡‡ | 71.5 ± 5.9 |
| VFA (cm2) | 16.3 (13.4–19.9) *,†,‡ | 17.9 (14.8–21.6) §,|| | 35.0 (25.1–47.3) ** | 35.4 (28.7–43.3) †† | 77.5 (57.7–87.1) | 72.4 (63.1–84.7) |
| LVEF% (%) | 67.4 ± 4.3 | 67.3 ± 4.1 | 68.2 ± 4.4 | 67.5 ± 4.4 | 68.2 ± 3.6 | 68.0 ± 4.5 |
| LVEDV (mL) | 56.1 ± 10.5 *,†,‡ | 50.2 ± 8.9 §,|| | 62.9 ± 10.0 ¶ | 55.6 ± 9.5 | 64.8 ± 12.2 | 60.4 ± 10.8 |
| LVESV (mL) | 18.2 ± 4.3 *,†,‡ | 16.4 ± 3.6 §,|| | 19.9 ± 3.7 ¶ | 18.1 ± 4.0 | 20.4 ± 4.5 | 19.2 ± 4.2 |
| LVM (g) | 61.3 ± 11.8 *,†,‡ | 52.5 ± 9.8 §, || | 71.6 ± 13.8 ¶,** | 60.9 ± 11.3 †† | 80.1 ± 15.8 ‡‡ | 70.4 ± 16.3 |
| LVM-indexed height (g/m) | 49.0 ± 8.8 *,†,‡ | 42.4 ± 7.4 §,|| | 55.9 ± 10.6 ¶,** | 47.9 ± 8.2 †† | 60.9 ± 11.0 ‡‡ | 54.2 ± 11.3 |
| LVM-indexed height2.7 (g/m2.7) | 33.5 ± 5.9 *,†,‡ | 29.5 ± 5.1 §,|| | 36.8 ± 7.5 ¶ | 31.9 ± 5.2 †† | 38.3 ± 6.6 ‡‡ | 34.9 ± 6.5 |
| Total cholesterol (mmol/L) | 4.3 (3.9–4.7) | 4.4 (3.9–4.7) | 4.1 (3.6–4.6) | 4.3 (4.0–4.6) | 4.5 (4.0–4.8) | 4.0 (3.7–4.8) |
| Fasting glucose (mmol/L) | 5.1 (4.8–5.3) * | 5.0 (4.7–5.2) | 5.1 (4.8–5.4) ¶ | 5.0 (4.8–5.2) | 5.2 (5.0–5.3) ‡‡ | 5.0 (4.9–5.1) |
| Sex | Variable | LVM | LVEDV | LVESV | LVM-Indexed Height | LVM-Indexed Height2.7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | ||
| Boys | Intercept | −8.44 | 14.86 | 0.57 | 13.37 | 13.08 | 0.31 | 1.25 | 5.28 | 0.81 | 13.91 | 11.16 | 0.21 | 33.32 | 7.66 | <0.001 |
| BMI | 2.94 | 0.39 | <0.001 | 2.29 | 0.34 | <0.001 | 0.70 | 0.14 | <0.001 | 2.21 | 0.29 | <0.001 | 1.35 | 0.20 | <0.001 | |
| Age | 1.96 | 1.82 | 0.28 | 1.30 | 1.61 | 0.42 | 0.80 | 0.65 | 0.22 | −0.20 | 1.37 | 0.89 | −2.17 | 0.94 | 0.022 | |
| SBP | 0.27 | 0.09 | 0.002 | 0.14 | 0.08 | 0.074 | 0.05 | 0.03 | 0.12 | 0.15 | 0.07 | 0.025 | 0.03 | 0.05 | 0.57 | |
| DBP | −0.29 | 0.11 | 0.009 | −0.25 | 0.10 | 0.012 | −0.07 | 0.04 | 0.096 | −0.21 | 0.08 | 0.012 | −0.11 | 0.06 | 0.046 | |
| VFA | −0.04 | 0.05 | 0.36 | −0.13 | 0.04 | 0.003 | −0.04 | 0.02 | 0.010 | −0.07 | 0.04 | 0.060 | −0.08 | 0.02 | 0.001 | |
| Girls | Intercept | −36.34 | 12.85 | 0.041 | −4.05 | 11.33 | 0.72 | 0.74 | 4.72 | 0.88 | −2.97 | 9.64 | 0.76 | 19.14 | 6.49 | 0.003 |
| BMI | 2.16 | 0.35 | <0.001 | 1.49 | 0.31 | <0.001 | 0.41 | 0.13 | 0.001 | 1.82 | 0.26 | <0.001 | 1.34 | 0.18 | <0.001 | |
| Age | 5.05 | 1.60 | 0.002 | 4.46 | 1.40 | 0.002 | 1.18 | 0.58 | 0.043 | 2.13 | 1.19 | 0.075 | −0.77 | 0.80 | 0.34 | |
| SBP | 0.07 | 0.08 | 0.35 | 0.08 | 0.07 | 0.25 | 0.02 | 0.03 | 0.51 | 0.02 | 0.06 | 0.74 | −0.03 | 0.04 | 0.42 | |
| DBP | 0.04 | 0.10 | 0.65 | −0.14 | 0.09 | 0.11 | −0.02 | 0.03 | 0.61 | 0.02 | 0.07 | 0.73 | 0.01 | 0.05 | 0.92 | |
| VFA | 0.04 | 0.05 | 0.37 | 0.01 | 0.04 | 0.85 | 0.01 | 0.02 | 0.70 | −0.03 | 0.03 | 0.44 | −0.09 | 0.02 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Shu, W.; Li, M.; Xu, L.; Amaerjiang, N.; Zunong, J.; Vermund, S.H.; Huang, D.; Chong, M.; Hu, Y. Sex-Specific Differences in Left Ventricular Mass and Volumes with Body Mass Index among Children Aged 6 to 8: A Cross-Sectional Study in China. Nutrients 2023, 15, 3066. https://doi.org/10.3390/nu15133066
Xiao H, Shu W, Li M, Xu L, Amaerjiang N, Zunong J, Vermund SH, Huang D, Chong M, Hu Y. Sex-Specific Differences in Left Ventricular Mass and Volumes with Body Mass Index among Children Aged 6 to 8: A Cross-Sectional Study in China. Nutrients. 2023; 15(13):3066. https://doi.org/10.3390/nu15133066
Chicago/Turabian StyleXiao, Huidi, Wen Shu, Menglong Li, Liyuan Xu, Nubiya Amaerjiang, Jiawulan Zunong, Sten H. Vermund, Dayong Huang, Mei Chong, and Yifei Hu. 2023. "Sex-Specific Differences in Left Ventricular Mass and Volumes with Body Mass Index among Children Aged 6 to 8: A Cross-Sectional Study in China" Nutrients 15, no. 13: 3066. https://doi.org/10.3390/nu15133066
APA StyleXiao, H., Shu, W., Li, M., Xu, L., Amaerjiang, N., Zunong, J., Vermund, S. H., Huang, D., Chong, M., & Hu, Y. (2023). Sex-Specific Differences in Left Ventricular Mass and Volumes with Body Mass Index among Children Aged 6 to 8: A Cross-Sectional Study in China. Nutrients, 15(13), 3066. https://doi.org/10.3390/nu15133066