The Variability of Vitamin D Concentrations in Short Children with Short Stature from Central Poland—The Effects of Insolation, Supplementation, and COVID-19 Pandemic Isolation
Abstract
:1. Introduction
- the seasonal variability of insolation,
- implementing new guidelines of vitamin D supplementation,
- the pandemic situation with limited sun exposure due to lockdown and strong recommendations to increase vitamin D supplementation on serum 25(OH)D concentrations in children.
2. Materials and Methods
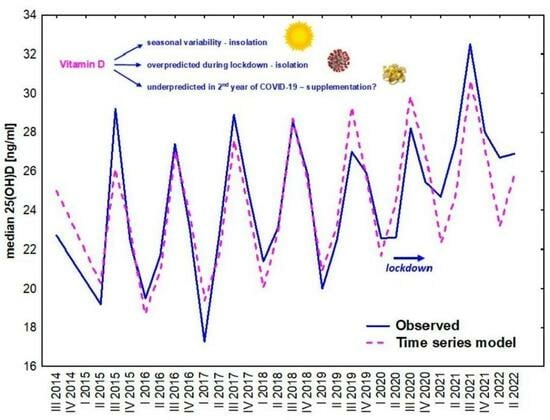
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66 (Suppl. S2), S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Wasiewicz, T.; Piotrowska, A.; Wierzbicka, J.; Slominski, A.T.; Zmijewski, M.A. Antiproliferative activity of non-calcemic vitamin D analogs on human melanoma lines in relation to VDR and PDIA3 receptors. Int. J. Mol. Sci. 2018, 19, 2583. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; de Girolamo, L.; Orfei, C.P.; Viganò, M.; Cecchinato, R.; Brayda-Bruno, M.; Colombini, A. Vitamin D’s effect on the proliferation and inflammation of human intervertebral disc cells in relation to the functional vitamin D receptor gene foki polymorphism. Int. J. Mol. Sci. 2018, 19, 2002. [Google Scholar] [CrossRef]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in COVID-19 infection in pediatric population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal changes in vitamin D-effective UVB availability in Europe and associations with population serum 25-hydroxyvitamin D. Nutrients 2016, 8, 553. [Google Scholar] [CrossRef]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Erba, P.; Saggese, G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Ital. J. Pediatr. 2014, 40, 54. [Google Scholar] [CrossRef]
- Oliosa, P.R.; Oliosa, E.M.R.; de Oliveira Alvim, R.; Sartório, C.L.; dos Anjos Zaniqueli, D.; Mill, J.G. Association of sun exposure and seasonality with vitamin D levels in Brazilian children and adolescents. Rev. Paul. Pediatr. 2023, 41, e2021361. [Google Scholar] [CrossRef]
- Pludowski, P.; Grant, W.B.; Bhattoa, H.P.; Bayer, M.; Povoroznyuk, V.; Rudenka, E.; Ramanau, H.; Varbiro, S.; Rudenka, A.; Karczmarewicz, E.; et al. Vitamin D status in central Europe. Int. J. Endocrinol. 2014, 2014, 589587. [Google Scholar] [CrossRef]
- Kiely, M.; Black, L.J. Dietary strategies to maintain adequacy of circulating 25-hydroxyvitamin D concentrations. Scand. J. Clin. Lab. Investig. 2012, 72, 14–23. [Google Scholar]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K.D. A systematic review of vitamin D status in southern European countries. Eur. J. Nutr. 2018, 57, 2001–2036. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Cashman, K.D. Food-based solutions for vitamin D deficiency: Putting policy into practice and the key role for research. Proc. Nutr. Soc. 2017, 76, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Vitamin D: Dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J. Steroid Biochem. Mol. Biol. 2015, 148, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Płudowski, P. COVID-19 and Other Pleiotropic Actions of Vitamin D: Proceedings from the Fifth International Conference “Vitamin D—Minimum, Maximum, Optimum” under the Auspices of the European Vitamin D Association (EVIDAS). Nutrients 2023, 15, 2530. [Google Scholar] [CrossRef]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Desci, T.; Franek, E.; Głuszko, P.; et al. Practical Guidelines for the Supplementation of Vitamin D and the Treatment of Deficits in Central Europe. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Chlebna-Sokół, D.; Michałus, I.; Rusińska, A.; Łupińska, A.; Fijałkowski, B.; Andrzejewska, K.; Magsar Khuchit, B.; Porczyński, M.; Woch, I.; Jończyk, A.; et al. Evaluation of vitamin D levels in children hospitalized with symptoms suggesting metabolism disorders in skeleton system. Pediatr. Endocrinol. 2016, 15, 23–32. [Google Scholar] [CrossRef]
- Smyczyńska, J.; Smyczyńska, U.; Stawerska, R.; Domagalska-Nalewajek, H.; Lewiński, A.; Hilczer, M. Seasonality of vitamin D concentrations and the incidence of vitamin D deficiency in children and adolescents from central Poland. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies—2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Szarpak, L.; Rafique, Z.; Gasecka, A.; Chirico, F.; Gawel, W.; Hernik, J.; Kaminska, H.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, Ł. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol. J. 2021, 28, 647–654. [Google Scholar] [CrossRef]
- Palczewska, I.; Niedźwiecka, Z. Indices of somatic development of children and adolescents in Warsaw. Med. Wieku Rozw. 2001, 5 (Suppl. S1), 17–118. (In Polish) [Google Scholar]
- Lin, L.; Ou, Q.; Lin, L.; Zhang, H.; Chen, K.; Chen, D.; Quan, H.; He, Y.; Fang, T. Low prevalence of vitamin D deficiency in adult residents in Hainan, the tropical island province of China. Ann. Palliat. Med. 2021, 10, 5580–6394. [Google Scholar] [CrossRef]
- Lee, J.; Woo, H.W.; Kim, J.; Shin, M.H.; Koh, I.; Choi, B.Y.; Kim, M.K. Independent and interactive associations of season, dietary vitamin D, and vitamin D-related genetic variants with serum 25(OH)D in Korean adults aged 40 years or older. Endocr. J. 2021, 68, 701–711. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Luzeiro, C.; Senise, J.F.; Castelo, A. Association of seasonality and serum albumin concentration with Vitamin D deficiency in subjects with chronic hepatitis C infection living in a sunny country. Public Health Nutr. 2020, 23, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Li, Z.; Lv, D.; Yang, G.; Wu, R.; Pan, J.; Wang, S.; Li, Y.; Xu, S. Seasonal variation and correlation analysis of vitamin D and parathyroid hormone in Hangzhou, Southeast China. J. Cell. Mol. Med. 2020, 24, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Dimakopoulos, I.; Magriplis, E.; Mitsopoulou, A.V.; Karageorgou, D.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; et al. Association of serum vitamin D status with dietary intake and sun exposure in adults. Clin. Nutr. ESPEN 2019, 34, 23–31. [Google Scholar] [CrossRef]
- Basińska-Lewandowska, M.; Lewiński, A.; Horzelski, W.; Skowrońska-Jóźwiak, E. Effect of summer sunshine exposure on vitamin D status in young and middle age Poles: Is 30 ng/ml vitamin D cut-off really suitable for the Polish population? Int. J. Environ. Res. Public Health 2021, 18, 8116. [Google Scholar] [CrossRef]
- Rustecka, A.; Maret, J.; Drab, A.; Leszczyńska, M.; Tomaszewska, A.; Lipińska-Opałka, A.; Będzichowska, A.; Kalicki, B.; Kubiak, J.Z. The impact of COVID-19 pandemic during 2020–2021 on the vitamin D serum levels in the paediatric population in Warsaw, Poland. Nutrients 2021, 13, 1990. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, N.; Kuwabara, A.; Ogasawara, H.; Nishino, M.; Nakagawa, K.; Kamao, M.; Hasegawa, H.; Tanaka, K. Vitamin D Status in Japanese Young Women in 2016–2017 and 2020: Seasonal Variation and the Effect of Lifestyle Including Changes Caused by the COVID-19 Pandemic. J. Nutr. Sci. Vitaminol. 2022, 68, 172–180. [Google Scholar] [CrossRef]
- Jastrzębska, J.; Skalska, M.; Radzimiński, Ł.; López-Sánchez, G.F.; Weiss, K.; Hill, L.; Knechtle, B. Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season. Nutrients 2022, 14, 521. [Google Scholar] [CrossRef]
- Lippi, G.; Ferrari, A.; Targher, G. Is COVID-19 lockdown associated with Vitamin D deficiency? Eur. J. Public Health 2021, 31, 278–279. [Google Scholar] [CrossRef]
- Ferrari, D.; Locatelli, M.; Faraldi, M.; Lombardi, G. Changes in 25-(OH) vitamin D levels during the SARS-CoV-2 outbreak: Lockdown-related effects and first-to-second wave difference—An observational study from northern Italy. Biology 2021, 10, 237. [Google Scholar] [CrossRef]
- Beyazgül, G.; Bağ, Ö.; Yurtseven, İ.; Coşkunol, F.; Başer, S.; Çiçek, D.; Kanberoğlu, G.İ.; Çelik, F.; Nalbantoğlu, Ö.; Özkan, B. How Vitamin D Levels of Children Changed During COVID-19 Pandemic: A Comparison of Pre-pandemic and Pandemic Periods. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2022, 14, 188–195. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Chen, N.; Yang, J.; Yang, J.; Bi, L. Influence of the COVID-19 pandemic on the vitamin D status of children: A cross-sectional study. J. Med. Virol. 2023, 95, e28438. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wei, Y.; Yue, X.; Xu, K.; Li, M.; Lin, W. Correlation analysis between the occurrence of epidemic in ancient China and solar activity. Sci. China Earth Sci. 2023, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Kim, J.S.; Kwak, Y.S. Relation of pandemics with solar cycles through ozone, cloud seeds, and vitamin D. Environ. Sci. Pollut. Res. 2022, 30, 13827–13836. [Google Scholar] [CrossRef] [PubMed]
- Bell, T. Do solar cycles explain the emergence of COVID-19? Neutron count comparison between the solar minima of 2008–2009 and 2019–2020. Curr. Opin. Environ. Sci. Health 2022, 26, 100333. [Google Scholar] [CrossRef]
- Fu, S.; Wang, B.; Zhou, J.; Xu, X.; Liu, J.; Ma, Y.; Li, L.; He, X.; Li, S.; Niu, J.; et al. Meteorological factors, governmental responses and COVID-19: Evidence from four European countries. Environ. Res. 2021, 194, 110596. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020, 724, 138201. [Google Scholar] [CrossRef]
- Ahmadi, M.; Sharifi, A.; Dorosti, S.; Jafarzadeh Ghoushchi, S.; Ghanbari, N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020, 729, 138705. [Google Scholar] [CrossRef]
- Şahin, M. Impact of weather on COVID-19 pandemic in Turkey. Sci. Total Environ. 2020, 728, 138810. [Google Scholar] [CrossRef]
- Loché Fernández-Ahúja, J.M.; Fernández Martínez, J.L. Effects of climate variables on the COVID-19 outbreak in Spain. Int. J. Hyg. Environ. Health 2021, 234, 113723. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Mutz, Y.S.; Bernardes, P.C.; Conte-Junior, C.A. Relationship between COVID-19 and weather: Case study in a tropical country. Int. J. Hyg. Environ. Health 2020, 229, 113587. [Google Scholar] [CrossRef]
- Basińska-Lewandowska, M.; Lewandowski, K.; Horzelski, W.; Lewiński, A.; Skowrońska-Jóźwiak, E. Frequency of COVID-19 Infection as a Function of Vitamin D Levels. Nutrients 2023, 15, 1581. [Google Scholar] [CrossRef]
- Borger, C.; Paolicelli, C.; Ritchie, L.; Whaley, S.E.; Dematteis, J.; Sun, B.; Zimmerman, T.P.; Reat, A.; Dixit-Joshi, S. Shifts in sources of food but stable nutritional outcomes among children in the early months of the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2021, 18, 12626. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J. Perspective: School Meal Programs Require Higher Vitamin D Fortification Levels in Milk Products and Plant-Based Alternatives—Evidence from the National Health and Nutrition Examination Surveys (NHANES 2001–2018). Adv. Nutr. 2022, 13, 1440–1449. [Google Scholar] [CrossRef]
- Mendes, M.M.; Hart, K.H.; Williams, E.L.; Mendis, J.; Lanham-New, S.A.; Botelho, P.B. Vitamin D supplementation and sunlight exposure on serum Vitamin D concentrations in 2 parallel, double-blind, randomized, placebo-controlled trials. J. Nutr. 2021, 151, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Field, C.J.; Weinberg, A.R.; Letourneau, N. Both mother and infant require a vitamin D supplement to ensure that infants’ vitamin D status meets current guidelines. Nutrients 2018, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, N.; Aghajafari, F.; Bell, R.C.; Deane, A.J.; Dewey, D.; Field, C.; Giesbrecht, G.; Kaplan, B.; Leung, B.; Ntanda, H. The Alberta Pregnancy Outcomes and Nutrition (APrON) longitudinal study: Cohort profile and key findings from the first three years. BMJ Open 2022, 12, e047503. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022, 13, 836738. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Mielech, A.; Markiewicz-Żukowska, R.; Mielcarek, K.; Moskwa, J.; Naliwajko, S.K.; Soroczyńska, J.; Gromkowska-Kępka, K.J.; et al. Consumption of food supplements during the three COVID-19 waves in Poland—Focus on zinc and vitamin D. Nutrients 2021, 13, 3361. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]




| Group | All | Low | Suboptimal | Optimal | High |
|---|---|---|---|---|---|
| No of patients (boys/girls) | 1440 (879/561) | 461 (285/176) | 604 (362/242) | 361 (223/138) | 14 (9/5) |
| Age [years] median (lower-upper quartile) | 10.0 | 11.1 a,b,c | 10.3 a,d | 8.6 b,d | 10.6 c |
| (6.9–12.9) | (8.5–13.6) | (7.1–12.8) | (5.5–12.0) | (6.2–11.3) |
| Group | All | Mid-2014 to Mid-2018 (Old Guidelines) | Mid-2018 to Mid-2020 (New Guidelines) | Mid-2020 to Mid-2022 (Pandemic) |
|---|---|---|---|---|
| No of patients (boys/girls) | 1397 (854/543) | 841 (501/340) | 314 (195/119) | 242 (158/84) |
| Age [years] median (lower-upper quartile) | 10.0 (7.0–12.9) | 10.1 (7.1–13.1) | 9.8 (6.8–12.6) | 9.9 (7.0–12.7) |
| 25(OH)D [ng/mL] (lower-upper quartile) | 24.2 | 22.9 a,b | 26.0 a,c | 29.9 b,c |
| (18.6–30.3) | (17.3–28.7) | (20.0–30.7) | (21.3–34.3) |
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|---|---|---|---|
| Winter | 2.4 | 1.6 | 2.2 | 2.0 | 1.7 | 2.1 | 2.2 | 1.8 | 2.1 |
| Spring | 5.0 | 4.3 | 4.3 | 4.3 | 5.5 | 4.2 | 5.3 | 4.5 | 5.6 |
| Summer | 6.8 | 8.0 | 8.5 | 7.9 | 9.0 | 8.6 | 8.0 | 8.2 | - |
| Autumn | 5.6 | 7.3 | 6.2 | 5.2 | 7.4 | 5.9 | 6.6 | 5.7 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyczyńska, J.; Pawelak, N.; Hilczer, M.; Łupińska, A.; Lewiński, A.; Stawerska, R. The Variability of Vitamin D Concentrations in Short Children with Short Stature from Central Poland—The Effects of Insolation, Supplementation, and COVID-19 Pandemic Isolation. Nutrients 2023, 15, 3629. https://doi.org/10.3390/nu15163629
Smyczyńska J, Pawelak N, Hilczer M, Łupińska A, Lewiński A, Stawerska R. The Variability of Vitamin D Concentrations in Short Children with Short Stature from Central Poland—The Effects of Insolation, Supplementation, and COVID-19 Pandemic Isolation. Nutrients. 2023; 15(16):3629. https://doi.org/10.3390/nu15163629
Chicago/Turabian StyleSmyczyńska, Joanna, Natalia Pawelak, Maciej Hilczer, Anna Łupińska, Andrzej Lewiński, and Renata Stawerska. 2023. "The Variability of Vitamin D Concentrations in Short Children with Short Stature from Central Poland—The Effects of Insolation, Supplementation, and COVID-19 Pandemic Isolation" Nutrients 15, no. 16: 3629. https://doi.org/10.3390/nu15163629
APA StyleSmyczyńska, J., Pawelak, N., Hilczer, M., Łupińska, A., Lewiński, A., & Stawerska, R. (2023). The Variability of Vitamin D Concentrations in Short Children with Short Stature from Central Poland—The Effects of Insolation, Supplementation, and COVID-19 Pandemic Isolation. Nutrients, 15(16), 3629. https://doi.org/10.3390/nu15163629