A Combination of Soy Isoflavone and L-Carnitine Improves Running Endurance in Mice
Abstract
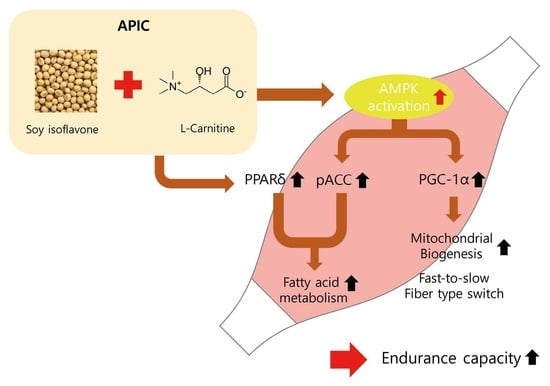
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Exhaustive Treadmill Test
2.4. Indirect Calorimetry and Body Composition
2.5. Immunofluorescence Staining
2.6. Cell Culture
2.7. Immunoblot Analysis
2.8. Quantitative PCR
2.9. Triglyceride Assay
2.10. ATP Assay
2.11. Measurement of Plasma Lactate Level
2.12. Measurement of Oxygen Consumption Rates
2.13. Statistical Analysis
3. Results
3.1. APIC Supplementation Enhances Running Endurance
3.2. APIC Supplementation Promotes the Transition from Glycolytic to Oxidative Myofibers
3.3. Effect of APIC Supplementation on Biochemical Parameters
3.4. APIC Supplementation Activates AMPK and Upregulates Downstream Target Proteins
3.5. APIC Supplementation Increases Mitochondrial Content and Oxidative Capacity
3.6. APIC Supplementation Increases the Expression of Genes and Proteins Involved in Fatty Acid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. Cmaj 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Spangenburg, E.E.; Booth, F.W. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol. Scand. 2003, 178, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, D.P.; Diffee, G.M.; Herrick, R.E.; Baldwin, K.M. Effects of endurance exercise on isomyosin patterns in fast- and slow-twitch skeletal muscles. J. Appl. Physiol. 1990, 68, 1950–1955. [Google Scholar] [CrossRef]
- Hall, E.C.R.; Semenova, E.A.; Bondareva, E.A.; Borisov, O.V.; Andryushchenko, O.N.; Andryushchenko, L.B.; Zmijewski, P.; Generozov, E.V.; Ahmetov, I.I. Association of muscle fiber composition with health and exercise-related traits in athletes and untrained subjects. Biol. Sport 2021, 38, 659–666. [Google Scholar] [CrossRef]
- Lee, Y.G.; Song, M.Y.; Cho, H.; Jin, J.S.; Park, B.H.; Bae, E.J. Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation. Nutrients 2022, 14, 3904. [Google Scholar] [CrossRef]
- Fan, W.; Waizenegger, W.; Lin, C.S.; Sorrentino, V.; He, M.X.; Wall, C.E.; Li, H.; Liddle, C.; Yu, R.T.; Atkins, A.R.; et al. PPARδ Promotes Running Endurance by Preserving Glucose. Cell Metab. 2017, 25, 1186–1193.e1184. [Google Scholar] [CrossRef]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Cha, Y.S.; Choi, S.K.; Suh, H.; Lee, S.N.; Cho, D.; Li, K. Effects of carnitine coingested caffeine on carnitine metabolism and endurance capacity in athletes. J. Nutr. Sci. Vitaminol. 2001, 47, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Pan, J.H.; Lee, E.S.; Kim, Y.J. L-Carnitine enhances exercise endurance capacity by promoting muscle oxidative metabolism in mice. Biochem. Biophys. Res. Commun. 2015, 464, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Colombani, P.; Wenk, C.; Kunz, I.; Krähenbühl, S.; Kuhnt, M.; Arnold, M.; Frey-Rindova, P.; Frey, W.; Langhans, W. Effects of L-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: A double-blind crossover field study. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 434–439. [Google Scholar] [CrossRef]
- Heinonen, O.J.; Takala, J.; Kvist, M.H. Effect of carnitine loading on long-chain fatty acid oxidation, maximal exercise capacity, and nitrogen balance. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Kim, Y.B.; Peroni, O.D.; Fryer, L.G.; Müller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758s–767s. [Google Scholar] [CrossRef]
- Kim, M.; Im, S.; Cho, Y.K.; Choi, C.; Son, Y.; Kwon, D.; Jung, Y.S.; Lee, Y.H. Anti-Obesity Effects of Soybean Embryo Extract and Enzymatically-Modified Isoquercitrin. Biomolecules 2020, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Palacios-González, B.; Zarain-Herzberg, A.; Flores-Galicia, I.; Noriega, L.G.; Alemán-Escondrillas, G.; Zariñan, T.; Ulloa-Aguirre, A.; Torres, N.; Tovar, A.R. Genistein stimulates fatty acid oxidation in a leptin receptor-independent manner through the JAK2-mediated phosphorylation and activation of AMPK in skeletal muscle. Biochim. Biophys. Acta 2014, 1841, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Eshima-Kondo, S.; et al. Isoflavones derived from soy beans prevent MuRF1-mediated muscle atrophy in C2C12 myotubes through SIRT1 activation. J. Nutr. Sci. Vitaminol. 2013, 59, 317–324. [Google Scholar] [CrossRef]
- Shin, E.S.; Cho, S.Y.; Lee, E.H.; Lee, S.J.; Chang, I.S.; Lee, T.R. Positive regulation of hepatic carnitine palmitoyl transferase 1A (CPT1A) activities by soy isoflavones and L-carnitine. Eur. J. Nutr. 2006, 45, 159–164. [Google Scholar] [CrossRef]
- Park, H.-W.; Yang, M.-S.; Lee, J.-H.; Shin, E.-S.; Kim, Y.; Chun, J.-Y.; Lee, T.-R.; Lee, S.-J. Long term feeding with soy isoflavone and L-carnitine synergistically suppresses body weight gain and adiposity in high-fat diet induced obese mice. Nutr. Sci. 2006, 9, 179–189. [Google Scholar]
- Im, H.; Lee, J.; Kim, K.; Son, Y.; Lee, Y.H. Anti-obesity effects of heat-transformed green tea extract through the activation of adipose tissue thermogenesis. Nutr. Metab. 2022, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion-Rivera, L.; Foltz, S.; Hartzell, H.C.; Choo, H. Myosoft: An automated muscle histology analysis tool using machine learning algorithm utilizing FIJI/ImageJ software. PLoS ONE 2020, 15, e0229041. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoon, Y.C.; Im, H.; Son, Y.; Kim, M.; Saha, A.; Choi, C.; Lee, J.; Lee, S.; Kim, J.H.; et al. Adipocyte lysoplasmalogenase TMEM86A regulates plasmalogen homeostasis and protein kinase A-dependent energy metabolism. Nat. Commun. 2022, 13, 4084. [Google Scholar] [CrossRef]
- Son, Y.; Choi, C.; Saha, A.; Park, J.H.; Im, H.; Cho, Y.K.; Seong, J.K.; Burl, R.B.; Rondini, E.A.; Granneman, J.G.; et al. REEP6 knockout leads to defective β-adrenergic signaling in adipocytes and promotes obesity-related metabolic dysfunction. Metabolism 2022, 130, 155159. [Google Scholar] [CrossRef]
- Correia, J.C.; Kelahmetoglu, Y.; Jannig, P.R.; Schweingruber, C.; Shvaikovskaya, D.; Zhengye, L.; Cervenka, I.; Khan, N.; Stec, M.; Oliveira, M.; et al. Muscle-secreted neurturin couples myofiber oxidative metabolism and slow motor neuron identity. Cell Metab. 2021, 33, 2215–2230.e2218. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Jiménez, A.; Hernández-Torres, R.P.; Torres-Durán, P.V.; Romero-Gonzalez, J.; Mascher, D.; Posadas-Romero, C.; Juárez-Oropeza, M.A. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin. Med. Circ. Respir. Pulm. Med. 2008, 2, 1–9. [Google Scholar] [CrossRef]
- Hurley, B.F.; Nemeth, P.M.; Martin, W.H., 3rd; Hagberg, J.M.; Dalsky, G.P.; Holloszy, J.O. Muscle triglyceride utilization during exercise: Effect of training. J. Appl. Physiol. 1986, 60, 562–567. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kühne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef]
- Fan, W.; Evans, R. PPARs and ERRs: Molecular mediators of mitochondrial metabolism. Curr. Opin. Cell Biol. 2015, 33, 49–54. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamamoto, J.; Iwasaki, S.; Asaba, H.; Hamura, H.; Ikeda, Y.; Watanabe, M.; Magoori, K.; Ioka, R.X.; Tachibana, K.; et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA 2003, 100, 15924–15929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhang, C.L.; Yu, R.T.; Cho, H.K.; Nelson, M.C.; Bayuga-Ocampo, C.R.; Ham, J.; Kang, H.; Evans, R.M. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004, 2, e294. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Barnes, S. Isoflavones and PPAR Signaling: A Critical Target in Cardiovascular, Metastatic, and Metabolic Disease. PPAR Res. 2010, 2010, 153252. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, S.J.; Park, H.W.; Cha, Y.S. Effect of genistein with carnitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high-fat diet. J. Med. Food 2006, 9, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef]
- Suetsugi, M.; Su, L.; Karlsberg, K.; Yuan, Y.C.; Chen, S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol. Cancer Res. 2003, 1, 981–991. [Google Scholar]









| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| Ppia | GTGGTCTTTGGGAAGGTGAA | TTACAGGACATTGCGAGCAG |
| Ppargc1a | ACACCTGTGACGCTTTCGCTG | AAGGACACGCTGTCCCATGA |
| Ppard | GGCAGCCTCAACATGGAATG | TCGAGCTTCATGCGGATTGT |
| Cpt1b | ATGCTCCGAGGCATTTGTCA | GGTCAGCTGGCCATGGTATT |
| Acadl | GGCGATTTCTGCCTGTGAGTTC | GCTGTCCACAAAAGCTCTGGTG |
| Pdk4 | GTCGAGCATCAAGAAAACCGTCC | GCGGTCAGTAATCCTCAGAGGA |
| Lipe | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| Lpl | GCGTAGCAGGAAGTCTGACCAA | AGCGTCATCAGGAGAAAGGCGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Joh, Y.; Choi, C.; Kim, K.; Lee, Y.-H. A Combination of Soy Isoflavone and L-Carnitine Improves Running Endurance in Mice. Nutrients 2023, 15, 3678. https://doi.org/10.3390/nu15173678
Lee J, Joh Y, Choi C, Kim K, Lee Y-H. A Combination of Soy Isoflavone and L-Carnitine Improves Running Endurance in Mice. Nutrients. 2023; 15(17):3678. https://doi.org/10.3390/nu15173678
Chicago/Turabian StyleLee, Jaewon, Yoonjoe Joh, Cheoljun Choi, Kyungmin Kim, and Yun-Hee Lee. 2023. "A Combination of Soy Isoflavone and L-Carnitine Improves Running Endurance in Mice" Nutrients 15, no. 17: 3678. https://doi.org/10.3390/nu15173678
APA StyleLee, J., Joh, Y., Choi, C., Kim, K., & Lee, Y. -H. (2023). A Combination of Soy Isoflavone and L-Carnitine Improves Running Endurance in Mice. Nutrients, 15(17), 3678. https://doi.org/10.3390/nu15173678