Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life
Abstract
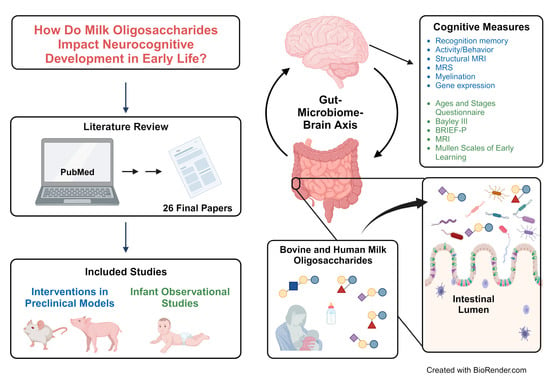
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
3. Results
3.1. Study Selection
3.2. Study Characteristics
| Ref. | First Author and Year | Country | Subjects | Sample Size (n) | Study Duration | Intervention or Exposure |
|---|---|---|---|---|---|---|
| [51] | Tarr, 2015 | US | Mice; male C57/BL6 | 54 |
Upon arrival for 20 days | Diets:
|
| [52] | Jacobi, 2016 | US | Pigs | 54 | 21 days | Diets:
|
| [53] | Oliveros, 2016 | Spain | Rat pups; Lister Hooded & Sprague-Dawley | 60 (Lister Hooded) & 60 (Sprague-Dawley) | PND 3 until weaning | Diets:
|
| [54] | Mudd, 2017 | US | Pigs; vaginally delivered male | 38 | PND 2 until PND 32 or 33 | Diets:
|
| [55] | Oliveros, 2018 | Spain | Rats; Sprague-Dawley | 30 | PND 3 until weaning (PND 22) | Diets:
|
| [56] | Fleming, 2018 | US | Pigs; naturally farrowed male | 36 | PND 2 until PND 22 | Diets:
|
| [57] | Obelitz-Ryom, 2019 | Denmark | Piglets; male & female | 40 (preterm), 14 (term, C-section), 12 (term, vaginal) | PND 0 until PND19 | Diets:
|
| [58] | Wang, 2019 | Australia | Piglets; domestic Sus scrofa | 46 |
PND 3 until PND 38 | Diets:
|
| [59] | Lee, 2020 | US | Mice; C57/BL6 male | 36 |
Six weeks of age until 12 weeks | Diets:
|
| [60] | Fleming, 2020 | US | Pigs; male | 36 |
PND 2 until PND 33 | Diets:
|
| [61] | Fleming, 2020 | US | Pigs; male | 48 |
PND 2 until PND 33 | Diets:
|
| [62] | Tuplin, 2021 | Canada | Rats; Sprague-Dawley both sex | 40 | PND 1 for Eight weeks | Diets:
|
| [63] | Pisa, 2021 | Italy | Mice; male | 28 | PND 0 until 23 weeks | Genotypes:
|
| [64] | Hauser, 2021 | Italy | Mice; male | 146 | PND 0 until 25 weeks | Genotypes:
|
| [65] | Clouard, 2021 | Denmark | Göttingen minipigs; female | 64 |
Two weeks until 45 weeks | Diets:
|
| [66] | Lee, 2021 | US | Mice; C57BL/6J male | 32 |
Six weeks until 14 weeks | Diets:
|
| [67] | Sutkus, 2022 | US | Pigs; male | 52 | PND 2 until PND 34 or 35 | Diets:
|
| [68] | Pisa, 2023 | Italy | Mice; both sex | 46 (Expt 1) 48 (Expt 2) | PND 0 until 25 weeks | Genotype:
|
| Ref. | First Author and Year | Country | Subjects | Sample Size | Study Duration | Exposure |
|---|---|---|---|---|---|---|
| [69] | Berger, 2020 | US | Hispanic mother-term infant dyads (males and females) | 50 |
| 19 HMO concentrations |
| [70] | Cho, 2021 | US | Mother-term infant dyads (males and females) | 99 |
| Eight HMO concentrations |
| [71] | Oliveros, 2021 | Spain | Normal weight, overweight, obese, and GDM mother-term infant dyads (males and females) | 82 |
| Two HMO concentrations |
| [72] | Jorgensen, 2021 | Malawi | Mother-term infant dyads (males and females) | 659 |
| 51 HMO relative abundances |
| [73] | Ferreira, 2021 | Brazil | Mother-term infant dyads (males and females) | 73 |
| 19 HMO concentrations |
| [74] | Rozé, 2022 | France | Mother-preterm infants dyads (males and females) | 137 |
| 24 HMO and total sialic acid concentrations for mean impute values over samples from 7 weeks |
| [75] | Berger, 2022 | US | Mother-term infant dyads (males and females) | 20 |
| 19 HMO concentrations |
| [76] | Willemsen, 2023 | The Netherlands | Mother-term infant dyads (males and females) | 63 |
| 24 HMO concentrations |
3.3. Sialyllactose and Cognition
3.3.1. Term and Preterm Piglet Models
3.3.2. Rodent Models
3.4. Fucosyllactose and Cognition
3.4.1. Piglet Models
3.4.2. Rodent Models
3.5. Human Studies on HMOS and Cognition
4. Discussion
4.1. Sialylated MOS and Cognition
4.2. Fucosylated MOS and Cognition
4.3. HMOS and Infant Cognition
4.4. Potential Mechanisms of MO Functions in Cognition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatnagar, S.; Taneja, S. Zinc and cognitive development. Br. J. Nutr. 2001, 85, S139–S145. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001, 2, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Pritchard, V.E.; Woodward, L.J. Preschool executive functioning abilities predict early mathematics achievement. Dev. Psychol. 2010, 46, 1176. [Google Scholar] [CrossRef]
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef]
- Martorell, R. Undernutrition during pregnancy and early childhood and its consequences for cognitive and behavioral development. In Early Child Development: Investing in Our Children’s Future; Young, M.E., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1997; pp. 39–83. [Google Scholar]
- Engle, P.L.; Fernández, P.D. INCAP studies of malnutrition and cognitive behavior. Food Nutr. Bull. 2010, 31, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Breastfeeding, S.O.; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Young, B. Breastfeeding and human milk: Short and long-term health benefits to the recipient infant. In Early Nutrition and Long-Term Health; Woodhead Publishing: Sawston, UK, 2017; pp. 25–53. [Google Scholar]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Horta, B.; Loret de Mola, C.; Victora, C. Breastfeeding and intelligence: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 14–19. [Google Scholar] [CrossRef]
- Bernard, J.Y.; De Agostini, M.; Forhan, A.; Alfaiate, T.; Bonet, M.; Champion, V.; Kaminski, M.; de Lauzon-Guillain, B.; Charles, M.-A.; Heude, B. Breastfeeding duration and cognitive development at 2 and 3 years of age in the EDEN mother–child Cohort. J. Pediatr. 2013, 163, 36–42.e31. [Google Scholar] [CrossRef]
- Angelsen, N.; Vik, T.; Jacobsen, G.; Bakketeig, L. Breast feeding and cognitive development at age 1 and 5 years. Arch. Dis. Child. 2001, 85, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Morley, R.; Cole, T.J. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ 1998, 317, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Chiurazzi, M.; Cozzolino, M.; Reinelt, T.; Nguyen, T.D.; Elke Chie, S.; Natalucci, G.; Miletta, M.C. Human milk and brain development in infants. Reprod. Med. 2021, 2, 107–117. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, H.; Fang, J.; Zhang, X. Enzymatic and chemoenzymatic synthesis of human milk oligosaccharides and derivatives. Carbohydr. Polym. 2022, 291, 119564. [Google Scholar] [CrossRef]
- Tao, N.; DePeters, E.; Freeman, S.; German, J.; Grimm, R.; Lebrilla, C.B. Bovine milk glycome. J. Dairy Sci. 2008, 91, 3768–3778. [Google Scholar] [CrossRef]
- Urashima, T.; Taufik, E.; Fukuda, K.; Asakuma, S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; Barile, D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv. Nutr. 2011, 2, 284–289. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M. Human milk oligosaccharides: 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) in infant formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Parschat, K.; Melsaether, C.; Jäpelt, K.R.; Jennewein, S. Clinical evaluation of 16-week supplementation with 5HMO-mix in healthy-term human infants to determine tolerability, safety, and effect on growth. Nutrients 2021, 13, 2871. [Google Scholar] [CrossRef]
- Lasekan, J.; Choe, Y.; Dvoretskiy, S.; Devitt, A.; Zhang, S.; Mackey, A.; Wulf, K.; Buck, R.; Steele, C.; Johnson, M. Growth and gastrointestinal tolerance in healthy term infants fed milk-based infant formula supplemented with five human milk oligosaccharides (HMOs): A randomized multicenter trial. Nutrients 2022, 14, 2625. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yeung, C.-Y. Recent advance in infant nutrition: Human milk oligosaccharides. Pediatr. Neonatol. 2021, 62, 347–353. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203. [Google Scholar]
- LeMay-Nedjelski, L.; Yonemitsu, C.; Asbury, M.R.; Butcher, J.; Ley, S.H.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W. Oligosaccharides and microbiota in human milk are interrelated at 3 months postpartum in a cohort of women with a high prevalence of gestational impaired glucose tolerance. J. Nutr. 2021, 151, 3431–3441. [Google Scholar] [CrossRef]
- Singh, R.P.; Niharika, J.; Kondepudi, K.K.; Bishnoi, M.; Tingirikari, J.M.R. Recent understanding of human milk oligosaccharides in establishing infant gut microbiome and roles in immune system. Food Res. Int. 2022, 151, 110884. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef]
- Martín-Sosa, S.; Martín, M.-J.; García-Pardo, L.-A.; Hueso, P. Sialyloligosaccharides in human and bovine milk and in infant formulas: Variations with the progression of lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef]
- Monaco, M.H.; Wang, M.; Pan, X.; Li, Q.; Richards, J.D.; Chichlowski, M.; Berg, B.M.; Dilger, R.N.; Donovan, S.M. Evaluation of sialyllactose supplementation of a prebiotic-containing formula on growth, intestinal development, and bacterial colonization in the neonatal piglet. Curr. Dev. Nutr. 2018, 2, nzy067. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, L.; Li, W.; Ni, D.; Zhang, W.; Yan, X.; Mu, W. Recent advances on 2′-fucosyllactose: Physiological properties, applications, and production approaches. Crit. Rev. Food Sci. Nutr. 2022, 62, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Mountford, C.; Quadrelli, S.; Lin, A.; Ramadan, S. Six fucose-α (1–2) sugars and α-fucose assigned in the human brain using in vivo two-dimensional MRS. NMR Biomed. 2015, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Matthies, H.; Staak, S.; Krug, M. Fucose and fucosyllactose enhance in-vitro hippocampal long-term potentiation. Brain Res. 1996, 725, 276–280. [Google Scholar] [CrossRef]
- Tosh, N.; Quadrelli, S.; Galloway, G.; Mountford, C. Two new fucose-α (1–2)-glycans assigned in the healthy human brain taking the number to seven. Sci. Rep. 2019, 9, 18806. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, W.; Popov, N.; Lössner, B.; Schulzeck, S.; Honza, R.; Matthies, H. Effect of L-fucose on brain protein metabolism and retention of a learned behavior in rats. Pharmacol. Biochem. Behav. 1980, 13, 765–771. [Google Scholar] [CrossRef]
- Kelly, R.J.; Rouquier, S.; Giorgi, D.; Lennon, G.G.; Lowe, J.B. Sequence and expression of a candidate for the human secretor blood group A (1, 2) fucosyltransferase gene (Fut2): Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 1995, 270, 4640–4649. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Borsch, C.; Vazquez, E.; Buck, R.; Reutzel, M.; Eckert, G.P.; Rudloff, S. Metabolic fate and distribution of 2-fucosyllactose: Direct influence on gut microbial activity but not on brain. Mol. Nutr. Food Res. 2019, 63, 1900035. [Google Scholar] [CrossRef]
- Rudloff, S.; Kuntz, S.; Borsch, C.; Vazquez, E.; Buck, R.; Reutzel, M.; Eckert, G.P.; Kunz, C. Fucose as a cleavage product of 2′Fucosyllactose does not cross the blood-brain barrier in mice. Mol. Nutr. Food Res. 2021, 65, 2100045. [Google Scholar] [CrossRef]
- Docq, S.; Spoelder, M.; Wang, W.; Homberg, J.R. The protective and long-lasting effects of human milk oligosaccharides on cognition in mammals. Nutrients 2020, 12, 3572. [Google Scholar] [CrossRef]
- Berger, P.K.; Ong, M.L.; Bode, L.; Belfort, M.B. Human Milk Oligosaccharides and Infant Neurodevelopment: A Narrative Review. Nutrients 2023, 15, 719. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, e55718. [Google Scholar]
- Silvers, J.M.; Harrod, S.B.; Mactutus, C.F.; Booze, R.M. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods 2007, 166, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Goulart, B.; De Lima, M.; De Farias, C.; Reolon, G.; Almeida, V.; Quevedo, J.; Kapczinski, F.; Schröder, N.; Roesler, R. Ketamine impairs recognition memory consolidation and prevents learning-induced increase in hippocampal brain-derived neurotrophic factor levels. Neuroscience 2010, 167, 969–973. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- d’Isa, R.; Comi, G.; Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Sci. Rep. 2021, 11, 21177. [Google Scholar] [CrossRef] [PubMed]
- Wenk, G.L. Assessment of spatial memory using the T maze. Curr. Protoc. Neurosci. 1998, 4, 8.5 B. 1–8.5 A. 7. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3’ Sialyllactose and 6’ Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula-fed piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2′-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef]
- Mudd, A.T.; Fleming, S.A.; Labhart, B.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary sialyllactose influences sialic acid concentrations in the prefrontal cortex and magnetic resonance imaging measures in corpus callosum of young pigs. Nutrients 2017, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary sialyllactose does not influence measures of recognition memory or diurnal activity in the young pig. Nutrients 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Obelitz-Ryom, K.; Bering, S.B.; Overgaard, S.H.; Eskildsen, S.F.; Ringgaard, S.; Olesen, J.L.; Skovgaard, K.; Pankratova, S.; Wang, B.; Brunse, A. Bovine milk oligosaccharides with sialyllactose improves cognition in preterm pigs. Nutrients 2019, 11, 1335. [Google Scholar] [CrossRef]
- Wang, H.X.; Chen, Y.; Haque, Z.; de Veer, M.; Egan, G.; Wang, B. Sialylated milk oligosaccharides alter neurotransmitters and brain metabolites in piglets: An In vivo magnetic resonance spectroscopic (MRS) study. Nutr. Neurosci. 2021, 24, 885–895. [Google Scholar] [CrossRef]
- Lee, S.; Goodson, M.; Vang, W.; Kalanetra, K.; Barile, D.; Raybould, H. 2′-fucosyllactose supplementation improves gut-brain signaling and diet-induced obese phenotype and changes the gut microbiota in high fat-fed mice. Nutrients 2020, 12, 1003. [Google Scholar] [CrossRef]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Dietary oligofructose alone or in combination with 2′-fucosyllactose differentially improves recognition memory and hippocampal mrna expression. Nutrients 2020, 12, 2131. [Google Scholar] [CrossRef]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Human and bovine milk oligosaccharides elicit improved recognition memory concurrent with alterations in regional brain volumes and hippocampal mRNA expression. Front. Neurosci. 2020, 14, 770. [Google Scholar] [CrossRef]
- Tuplin, E.W.N.; Chleilat, F.; Alukic, E.; Reimer, R.A. The Effects of Human Milk Oligosaccharide Supplementation During Critical Periods of Development on the Mesolimbic Dopamine System. Neuroscience 2021, 459, 166–178. [Google Scholar] [CrossRef]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macrì, S. Exposure to 3’ Sialyllactose-Poor Milk during Lactation Impairs Cognitive Capabilities in Adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Pisa, E.; Arias Vásquez, A.; Tomasi, F.; Traversa, A.; Chiodi, V.; Martin, F.-P.; Sprenger, N.; Lukjancenko, O.; Zollinger, A. Sialylated human milk oligosaccharides program cognitive development through a non-genomic transmission mode. Mol. Psychiatry 2021, 26, 2854–2871. [Google Scholar] [CrossRef] [PubMed]
- Clouard, C.; Reimert, I.; Fleming, S.A.; Koopmans, S.-J.; Schuurman, T.; Hauser, J. Dietary sialylated oligosaccharides in early-life may promote cognitive flexibility during development in context of obesogenic dietary intake. Nutr. Neurosci. 2021, 25, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goodson, M.L.; Vang, W.; Rutkowsky, J.; Kalanetra, K.; Bhattacharya, M.; Barile, D.; Raybould, H.E. Human milk oligosaccharide 2′-fucosyllactose supplementation improves gut barrier function and signaling in the vagal afferent pathway in mice. Food Funct. 2021, 12, 8507–8521. [Google Scholar] [CrossRef] [PubMed]
- Sutkus, L.T.; Joung, S.; Hirvonen, J.; Jensen, H.M.; Ouwehand, A.C.; Mukherjea, R.; Donovan, S.M.; Dilger, R.N. Influence of 2′-Fucosyllactose and Bifidobacterium longum Subspecies infantis Supplementation on Cognitive and Structural Brain Development in Young Pigs. Front. Neurosci. 2022, 16, 860368. [Google Scholar] [CrossRef]
- Pisa, E.; Traversa, A.; Caputo, V.; Ottomana, A.M.; Hauser, J.; Macrì, S. Long-term consequences of reduced availability and compensatory supplementation of sialylated HMOs on cognitive capabilities. Front. Cell. Neurosci. 2023, 17, 1091890. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Cho, S.; Zhu, Z.; Li, T.; Baluyot, K.; Howell, B.R.; Hazlett, H.C.; Elison, J.T.; Hauser, J.; Sprenger, N.; Wu, D. Human milk 3′-Sialyllactose is positively associated with language development during infancy. Am. J. Clin. Nutr. 2021, 114, 588–597. [Google Scholar] [CrossRef]
- Oliveros, E.; Martín, M.; Torres-Espínola, F.; Segura-Moreno, M.; Ramírez, M.; Santos, A.; Buck, R.; Rueda, R.; Escudero, M.; Catena, A. Human milk levels of 2-fucosyllactose and 6-sialyllactose are positively associated with infant neurodevelopment and are not impacted by maternal BMI or diabetic status. J. Nutr. Food Sci. 2021, 4, 100024. [Google Scholar]
- Jorgensen, J.M.; Young, R.; Ashorn, P.; Ashorn, U.; Chaima, D.; Davis, J.C.; Goonatilleke, E.; Kumwenda, C.; Lebrilla, C.B.; Maleta, K. Associations of human milk oligosaccharides and bioactive proteins with infant growth and development among Malawian mother-infant dyads. Am. J. Clin. Nutr. 2021, 113, 209–220. [Google Scholar] [CrossRef]
- Ferreira, A.L.L.; Alves-Santos, N.H.; Freitas-Costa, N.C.; Santos, P.P.; Batalha, M.A.; Figueiredo, A.C.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L. Associations Between Human Milk Oligosaccharides at 1 Month and Infant Development Throughout the First Year of Life in a Brazilian Cohort. J. Nutr. 2021, 151, 3543–3554. [Google Scholar] [CrossRef] [PubMed]
- Rozé, J.-C.; Hartweg, M.; Simon, L.; Billard, H.; Chen, Y.; Austin, S.; Boscher, C.; Moyon, T.; Darmaun, D.; Rodenas, C.L.G. Human milk oligosaccharides in breast milk and 2-year outcome in preterm infants: An exploratory analysis. Clin. Nutr. 2022, 41, 1896–1905. [Google Scholar] [CrossRef]
- Berger, P.K.; Bansal, R.; Sawardekar, S.; Yonemitsu, C.; Furst, A.; Hampson, H.E.; Schmidt, K.A.; Alderete, T.L.; Bode, L.; Goran, M.I. Associations of human milk oligosaccharides with infant brain tissue organization and regional blood flow at 1 month of age. Nutrients 2022, 14, 3820. [Google Scholar] [CrossRef]
- Willemsen, Y.; Beijers, R.; Gu, F.; Vasquez, A.A.; Schols, H.A.; de Weerth, C. Fucosylated human milk oligosaccharides during the first 12 postnatal weeks are associated with better executive functions in toddlers. Nutrients 2023, 15, 1463. [Google Scholar] [CrossRef] [PubMed]
- McVeagh, P.; Miller, J.B. Human milk oligosaccharides: Only the breast. J. Paediatr. Child Health 1997, 33, 281–286. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F.; Allergens, F.; Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of 6′-sialyllactose (6′-SL) sodium salt produced by derivative strains of Escherichia coli BL21 (DE3) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07645. [Google Scholar]
- Gieling, E.T.; Schuurman, T.; Nordquist, R.E.; van der Staay, F.J. The pig as a model animal for studying cognition and neurobehavioral disorders. Mol. Funct. Model. Neuropsychiatry 2011, 7, 359–383. [Google Scholar]
- Odle, J.; Lin, X.; Jacobi, S.K.; Kim, S.W.; Stahl, C.H. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu. Rev. Anim. Biosci. 2014, 2, 419–444. [Google Scholar] [CrossRef]
- Mudd, A.T.; Dilger, R.N. Early-life nutrition and neurodevelopment: Use of the piglet as a translational model. Adv. Nutr. 2017, 8, 92–104. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, L.J.; van der Ham, I.J. How does the corpus callosum mediate interhemispheric transfer? A review. Behav. Brain Res. 2011, 223, 211–221. [Google Scholar] [CrossRef]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; Mc Jarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef]
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatr. 2014, 14, 306. [Google Scholar] [CrossRef]
- Singh, A.; Yeh, C.J.; Blanchard, S.B. Ages and stages questionnaire: A global screening scale. Boletín Médico Del Hosp. Infant. De México 2017, 74, 5–12. [Google Scholar]
- Müller, U.; Kerns, K. The development of executive function. In Handbook of Child Psychology and Developmental Science; Lerner, R.R.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–53. [Google Scholar]
- Blair, C.; Razza, R.P. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007, 78, 647–663. [Google Scholar] [CrossRef] [PubMed]
- San Diego, R.J.; Franke, N.; Harding, J.E.; Wouldes, T.A. Cross-cultural validity and reliability of the BRIEF-P at age 2 and 4.5 years in children born at risk of neonatal hypoglycemia. Child Neuropsychol. 2023, 29, 340–356. [Google Scholar] [CrossRef]
- Nejati, V.; Salehinejad, M.A.; Nitsche, M.A.; Najian, A.; Javadi, A.-H. Transcranial direct current stimulation improves executive dysfunctions in ADHD: Implications for inhibitory control, interference control, working memory, and cognitive flexibility. J. Atten. Disord. 2020, 24, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Heine, W.; Wutzke, K.; Radke, M. Sialic acid in breast milk and infant formula food. Mon. Kinderheilkd. 1993, 141, 946–950. [Google Scholar]
- Zhang, X.; Liu, Y.; Liu, L.; Li, J.; Du, G.; Chen, J. Microbial production of sialic acid and sialylated human milk oligosaccharides: Advances and perspectives. Biotechnol. Adv. 2019, 37, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic Acids: Chemistry, Metabolism, and Function; Springer Science & Business Media: Berlin, Germany, 2012; Volume 10. [Google Scholar]
- Bogoch, S. Recognins and their chemoreciprocals. In Behavioral Neurochemistry; Spectrum-Wiley Press: New York, NY, USA, 1977; p. 270. [Google Scholar]
- Schmidt, R. Glycoproteins Involved in Long Lasting Plasticity in the Teleost Brain. 1989. Available online: https://jlupub.ub.uni-giessen.de/bitstream/handle/jlupub/16860/SchmidtRupert-Glycoproteinsin.pdf?sequence=1&isAllowed=y (accessed on 4 April 2023).
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated oligosaccharides and glycoconjugates of human milk. The impact on infant and newborn protection, development and well-being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef]
- Scholtz, S.A.; Gottipati, B.S.; Gajewski, B.J.; Carlson, S.E. Dietary sialic acid and cholesterol influence cortical composition in developing rats. J. Nutr. 2013, 143, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Martin, M.J.; Prieto, P.A. 2′-Fucosyllactose: An abundant, genetically determined soluble glycan present in human milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-W.; Wu, K.-J.; Wang, Y.-S.; Bae, E.-K.; Song, Y.; Yoon, J.; Yu, S.-J. Human milk oligosaccharide 2′-fucosyllactose induces neuroprotection from intracerebral hemorrhage stroke. Int. J. Mol. Sci. 2021, 22, 9881. [Google Scholar] [CrossRef]
- Wu, K.-J.; Chen, Y.-H.; Bae, E.-K.; Song, Y.; Min, W.; Yu, S.-J. Human milk oligosaccharide 2′-fucosyllactose reduces neurodegeneration in stroke brain. Transl. Stroke Res. 2020, 11, 1001–1011. [Google Scholar] [CrossRef]
- Hayashi, Y. Molecular mechanism of hippocampal long-term potentiation–Towards multiscale understanding of learning and memory. Neurosci. Res. 2022, 175, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Teyler, T.J.; DiScenna, P. Long-term potentiation. Annu. Rev. Neurosci. 1987, 10, 131–161. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Krug, M.; Wagner, M.; Staak, S.; Smalla, K.-H. Fucose and fucose-containing sugar epitopes enhance hippocampal long-term potentiation in the freely moving rat. Brain Res. 1994, 643, 130–135. [Google Scholar] [CrossRef]
- Vazquez, E.; Barranco, A.; Ramirez, M.; Gruart, A.; Delgado-Garcia, J.M.; Jimenez, M.L.; Buck, R.; Rueda, R. Dietary 2′-fucosyllactose enhances operant conditioning and long-term potentiation via gut-brain communication through the vagus nerve in rodents. PLoS ONE 2016, 11, e0166070. [Google Scholar] [CrossRef]
- Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Martínez-Lara, E.; Blanco, S.; Martín, M.J.; Castanys, E.; Buck, R. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J. Nutr. Biochem. 2015, 26, 455–465. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bliss, T.V. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Bruner, J.S. The course of cognitive growth. Am. Psychol. 1964, 19, 1. [Google Scholar] [CrossRef]
- Rutter, M. Family and school influences on cognitive development. J. Child Psychol. Psychiatry 1985, 26, 683–704. [Google Scholar] [CrossRef] [PubMed]
- McMath, A.L.; Aguilar-Lopez, M.; Cannavale, C.N.; Khan, N.A.; Donovan, S.M. A systematic review on the impact of gastrointestinal microbiota composition and function on cognition in healthy infants and children. Front. Neurosci. 2023, 17, 1171970. [Google Scholar] [CrossRef]
- Hüppi, P.S.; Maier, S.E.; Peled, S.; Zientara, G.P.; Barnes, P.D.; Jolesz, F.A.; Volpe, J.J. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr. Res. 1998, 44, 584–590. [Google Scholar] [CrossRef]
- Dean, J.M.; McClendon, E.; Hansen, K.; Azimi-Zonooz, A.; Chen, K.; Riddle, A.; Gong, X.; Sharifnia, E.; Hagen, M.; Ahmad, T. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci. Transl. Med. 2013, 5, 168ra167. [Google Scholar] [CrossRef]
- Zhong, J.; Li, J.; Ni, C.; Zuo, Z. Amantadine alleviates postoperative cognitive dysfunction possibly by preserving neurotrophic factor expression and dendritic arborization in the hippocampus of old rodents. Front. Aging Neurosci. 2020, 12, 605330. [Google Scholar] [CrossRef] [PubMed]
- Knutson, D.; Mitzey, A.; Talton, L.; Clagett-Dame, M. Mice null for NEDD9 (HEF1) display extensive hippocampal dendritic spine loss and cognitive impairment. Brain Res. 2016, 1632, 141–155. [Google Scholar] [CrossRef]
- Filippi, C.G.; Watts, R.; Duy, L.A.; Cauley, K.A. Diffusion-tensor imaging derived metrics of the corpus callosum in children with neurofibromatosis type I. Am. J. Roentgenol. 2013, 200, 44–49. [Google Scholar] [CrossRef]
- Stiles, J.; Brown, T.T.; Haist, F.; Jernigan, T.L. Brain and cognitive development. In Handbook of Child Psychology and Developmental Science; John Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 1–54. [Google Scholar]
- Morales, E.; Bustamante, M.; Gonzalez, J.R.; Guxens, M.; Torrent, M.; Mendez, M.; Garcia-Esteban, R.; Julvez, J.; Forns, J.; Vrijheid, M. Genetic variants of the FADS gene cluster and ELOVL gene family, colostrums LC-PUFA levels, breastfeeding, and child cognition. PLoS ONE 2011, 6, e17181. [Google Scholar] [CrossRef]
- Caspi, A.; Williams, B.; Kim-Cohen, J.; Craig, I.W.; Milne, B.J.; Poulton, R.; Schalkwyk, L.C.; Taylor, A.; Werts, H.; Moffitt, T.E. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 18860–18865. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.D.; Davey Smith, G.; Emmett, P.M.; Hibbeln, J.R.; Golding, J. FADS2 polymorphisms modify the effect of breastfeeding on child IQ. PLoS ONE 2010, 5, e11570. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Dou, Y.; Luo, Y.; Xing, Y.; Liu, H.; Chen, B.; Zhu, L.; Ma, D.; Zhu, J. Human Milk Oligosaccharides Variation in Gestational Diabetes Mellitus Mothers. Nutrients 2023, 15, 1441. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Yellowhair, T.R.; Oppong, A.Y.; Camacho, J.E.; Lowe, J.R.; Jantzie, L.L.; Ohls, R.K. Cognitive development in preterm infants: Multifaceted deficits reflect vulnerability of rigorous neurodevelopmental pathways. Minerva Pediatr. 2017, 69, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.E.; Arslanoglu, S.; Bertino, E.; Corvaglia, L.; Montirosso, R.; Picaud, J.-C.; Polberger, S.; Schanler, R.J.; Steel, C.; van Goudoever, J. XII. Human milk in feeding premature infants: Consensus statement. J. Pediatr. Gastroenterol. Nutr. 2015, 61, S16–S19. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, S.; Bode, L. Human milk oligosaccharides and the preterm infant: A journey in sickness and in health. Clin. Perinatol. 2017, 44, 193–207. [Google Scholar] [CrossRef]
- Davis, E.C.; Wang, M.; Donovan, S.M. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes 2017, 8, 143–171. [Google Scholar] [CrossRef]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.-S.; German, J.B. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef]
- Savignac, H.; Tramullas, M.; Kiely, B.; Dinan, T.; Cryan, J. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Galuska, C.E.; Rudloff, S.; Kuntz, S.; Borsch, C.; Reutzel, M.; Eckert, G.; Galuska, S.P.; Kunz, C. Metabolic fate and organ distribution of 13C-3′-sialyllactose and 13C-N-acetylneuraminic acid in wild-type mice–No evidence for direct incorporation into the brain. J. Funct. Foods 2020, 75, 104268. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Wang, M.; Monaco, M.H.; Hauser, J.; Yan, J.; Dilger, R.N.; Donovan, S.M. Bovine milk oligosaccharides and human milk oligosaccharides modulate the gut microbiota composition and volatile fatty acid concentrations in a preclinical neonatal model. Microorganisms 2021, 9, 884. [Google Scholar] [CrossRef]
- Fleming, S.A.; Hauser, J.; Yan, J.; Donovan, S.M.; Wang, M.; Dilger, R.N. A mediation analysis to identify links between gut bacteria and memory in context of human milk oligosaccharides. Microorganisms 2021, 9, 846. [Google Scholar] [CrossRef]
- Keim, S.A.; Sullivan, J.A.; Sheppard, K.; Smith, K.; Ingol, T.; Boone, K.M.; Malloy-McCoy, A.; Oza-Frank, R. Feeding infants at the breast or feeding expressed human milk: Long-term cognitive, executive function, and eating behavior outcomes at age 6 years. J. Pediatr. 2021, 233, 66–73.e61. [Google Scholar] [CrossRef]
- Richards, M.; Hardy, R.; Wadsworth, M.E. Long-term effects of breast-feeding in a national birth cohort: Educational attainment and midlife cognitive function. Public Health Nutr. 2002, 5, 631–635. [Google Scholar] [CrossRef]
- Oddy, W.H.; Kendall, G.E.; Li, J.; Jacoby, P.; Robinson, M.; De Klerk, N.H.; Silburn, S.R.; Zubrick, S.R.; Landau, L.I.; Stanley, F.J. The long-term effects of breastfeeding on child and adolescent mental health: A pregnancy cohort study followed for 14 years. J. Pediatr. 2010, 156, 568–574. [Google Scholar] [CrossRef]
- Lenehan, S.M.; Boylan, G.B.; Livingstone, V.; Fogarty, L.; Twomey, D.M.; Nikolovski, J.; Irvine, A.D.; Kiely, M.; Kenny, L.C.; Hourihane, J.O. The impact of short-term predominate breastfeeding on cognitive outcome at 5 years. Acta Paediatr. 2020, 109, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S. Publication bias-Importance of studies with negative results! Indian J. Anaesth. 2019, 63, 505. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.-P. Does the contribution of human milk oligosaccharides to the beneficial effects of breast milk allow us to hope for an improvement in infant formulas? Crit. Rev. Food Sci. Nutr. 2021, 61, 1503–1514. [Google Scholar] [CrossRef] [PubMed]

| Gestation | Diet | Analyses | Outcome | Ref. |
|---|---|---|---|---|
| Preterm piglets; 90% gestation (day 106) & Term piglets; C-section & Term piglets; Vaginally delivered |
|
|
| [57] |
| Term pigs; PND2 |
|
|
| [56] |
| Term pigs; PND2 |
|
|
| [54] |
| Term pigs; PND3 |
|
|
| [58] |
| Term Göttingen minipigs; 2 weeks old |
|
|
| [65] |
| Term pigs; PND1 |
|
|
| [52] |
| Gestation | Diet | Analysis | Outcome | Ref. |
|---|---|---|---|---|
| Mice; PND 0 | Genotyping
|
|
| [63] |
| Mice; 6–8 weeks old |
|
|
| [51] |
| Mice; PND 0 | Genotyping
|
|
| [64] |
| Rats; PND 3 |
|
|
| [55] |
| Mice; PND 0 | Genotyping
|
| Experiment 1:
| [68] |
| Gestation | Diet | Test | Outcome | Ref. |
|---|---|---|---|---|
| Term pigs; PND 2 |
|
|
| [67] |
| Term pigs; PND 2 |
|
|
| [60] |
| Term pigs, PND 2 |
|
|
| [61] |
| Gestation | Diet | Analysis | Outcome | Ref. |
|---|---|---|---|---|
| Rats; PND 3 |
|
|
| [53] |
| Rats; PND21 |
|
|
| [62] |
| Mice; 6-week-old |
|
|
| [59] |
| Mice; 6-week-old |
|
|
| [66] |
| Maternal Condition | HMO Assessment | Covariates Adjusted | Tests | Outcome | Ref. |
|---|---|---|---|---|---|
| At least partially BF at the study visit |
|
|
|
| [70] |
| Hispanic mothers with pre-pregnancy normal or overweight Exclusively BF for six months |
|
| Bayley Scales of Infant Development (Bayley III) |
| [69] |
| Study groups: Healthy normal weight Overweight Obese GDM No information is available on BF |
|
| Bayley III |
| [71] |
| Exclusively BF for seven weeks |
|
| Ages and Stages Questionnaire (ASQ) |
| [74] |
| Healthy women 67.0% Exclusively BF at one month |
|
| Brazilian Ages and Stages Questionnaire (ASQ-BR) | Negative associations between:
| [73] |
| Women from the iLiNS project No information is available on BF |
|
|
|
| [72] |
| Healthy mothers in the Netherlands 71.4% Exclusively BF for 12 weeks |
|
|
|
Analyses with exclusively breastfed infants:
| [76] |
| Healthy mothers with full-term singleton birth Exclusively BF at one month |
|
|
| At one month postpartum: Negative associations between:
| [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; McMath, A.L.; Donovan, S.M. Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life. Nutrients 2023, 15, 3743. https://doi.org/10.3390/nu15173743
Fan Y, McMath AL, Donovan SM. Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life. Nutrients. 2023; 15(17):3743. https://doi.org/10.3390/nu15173743
Chicago/Turabian StyleFan, Yuting, Arden L. McMath, and Sharon M. Donovan. 2023. "Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life" Nutrients 15, no. 17: 3743. https://doi.org/10.3390/nu15173743
APA StyleFan, Y., McMath, A. L., & Donovan, S. M. (2023). Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life. Nutrients, 15(17), 3743. https://doi.org/10.3390/nu15173743