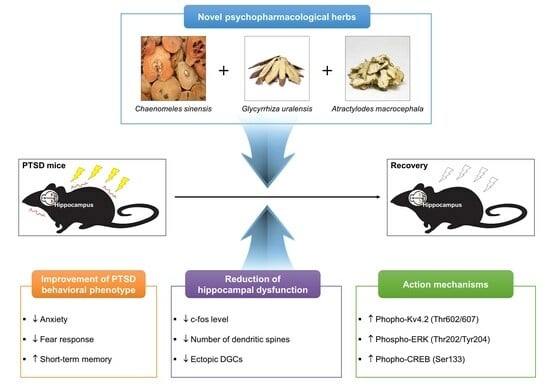
Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HFE
2.2. Establishment of PTSD Mice Model
2.3. Drug Administration
2.4. Open Field Test (OFT)
2.5. Y-Maze Test
2.6. Fear Response Test
2.7. Tissue Preparation
2.8. Serum Corticosterone Measurement
2.9. Immunostaining
2.10. Golgi Staining
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Effects of HFE Administration on Serum Corticosterone Levels in PTSD Mice
3.2. HFE Administration Attenuates PTSD-Like Behavioral Abnormalities
3.3. HFE Administration Protects against Abnormal Hippocampal Neurogenesis in PTSD Mice
3.4. HFE Administration Reduces Hilar Ectopic DGCs in PTSD Mice
3.5. HFE Administration Suppresses SPS+FS-Induced DGC Hyperactivation
3.6. HFE Restores the Activation of the Kv4.2/ERK/CREB Pathway Reduced by SPS+FS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Kheirbek, M.A.; Klemenhagen, K.C.; Sahay, A.; Hen, R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012, 15, 1613–1620. [Google Scholar]
- Lopresto, D.; Schipper, P.; Homberg, J.R. Neural circuits and mechanisms involved in fear generalization: Implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2016, 60, 31–42. [Google Scholar] [PubMed]
- Logue, M.W.; van Rooij, S.J.H.; Dennis, E.L.; Davis, S.L.; Hayes, J.P.; Stevens, J.S.; Densmore, M.; Haswell, C.C.; Ipser, J.; Koch, S.B.J.; et al. Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry 2018, 83, 244–253. [Google Scholar] [PubMed]
- Wang, Z.; Neylan, T.C.; Mueller, S.G.; Lenoci, M.; Truran, D.; Marmar, C.R.; Weiner, M.W.; Schuff, N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry 2010, 67, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Irle, E.; Ruhleder, M.; Lange, C.; Seidler-Brandler, U.; Salzer, S.; Dechent, P.; Weniger, G.; Leibing, E.; Leichsenring, F. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J. Psychiatry Neurosci. 2010, 35, 126–131. [Google Scholar] [CrossRef]
- Kitayama, N.; Vaccarino, V.; Kutner, M.; Weiss, P.; Bremner, J.D. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J. Affect Disord. 2005, 88, 79–86. [Google Scholar]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar]
- Hoschl, C.; Hajek, T. Hippocampal damage mediated by corticosteroids—A neuropsychiatric research challenge. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 81–88. [Google Scholar]
- Petrakis, I.L.; Simpson, T.L. Posttraumatic stress disorder and alcohol use disorder: A critical review of pharmacologic treatments. Alcohol. Clin. Exp. Res. 2017, 41, 226–237. [Google Scholar] [CrossRef]
- De Vries, Y.A.; de Jonge, P.; van den Heuvel, E.; Turner, E.H.; Roest, A.M. Influence of baseline severity on antidepressant efficacy for anxiety disorders: Meta-analysis and meta-regression. Br. J. Psychiatry 2016, 208, 515–521. [Google Scholar] [CrossRef]
- Jeffreys, M.; Capehart, B.; Friedman, M.J. Pharmacotherapy for posttraumatic stress disorder: Review with clinical applications. J. Rehabil. Res. Dev. 2012, 49, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Shergis, J.L.; Wu, L.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C. Herbal medicine for adults with asthma: A systematic review. J. Asthma. 2016, 53, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasmurthy, S.G.; Liu, J.Y.; Song, J.X.; Yang, C.B.; Malampati, S.; Wang, Z.Y.; Huang, Y.Y.; Li, M. Neurogenic traditional chinese medicine as a promising strategy for the treatment of alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 272. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, Z.; Zhao, L.; Wang, W.; Gao, M.; Jia, X.; Ouyang, H.; He, J. Anti-tumor activities and mechanisms of traditional Chinese medicines formulas: A review. Biomed. Pharmacother. 2020, 132, 110820. [Google Scholar]
- Cheon, C.; Ko, S.G. Synergistic effects of natural products in combination with anticancer agents in prostate cancer: A scoping review. Front. Pharmacol. 2022, 13, 963317. [Google Scholar] [PubMed]
- Enioutina, E.Y.; Salis, E.R.; Job, K.M.; Gubarev, M.I.; Krepkova, L.V.; Sherwin, C.M. Herbal medicines: Challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expert. Rev. Clin. Pharmacol. 2017, 10, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.F.; Zhou, W.J.; Wu, R.; Su, S.B. Systems biology approaches in the study of Chinese herbal formulae. Chin. Med. 2018, 13, 65. [Google Scholar] [PubMed]
- Cai, M.; Yang, E.J. Hochu-Ekki-To improves motor function in an amyotrophic lateral sclerosis animal model. Nutrients 2019, 11, 2644. [Google Scholar]
- Yang, E.J.; Lee, S.H.; Cai, M. Treatment with herbal formula extract in the hSOD1(G93A) mouse model attenuates muscle and spinal cord dysfunction via anti-inflammation. Mediators Inflamm. 2022, 2022, 4754732. [Google Scholar] [CrossRef]
- Burns, J.J.; Zhao, L.; Taylor, E.W.; Spelman, K. The influence of traditional herbal formulas on cytokine activity. Toxicology 2010, 278, 140–159. [Google Scholar] [PubMed]
- Amin, A.; Hossen, M.J.; Fu, X.Q.; Chou, J.Y.; Wu, J.Y.; Wang, X.Q.; Chen, Y.J.; Wu, Y.; Yin, C.L.; Dou, X.B.; et al. Inhibition of the Akt/NF-kappaB pathway is involved in the anti-gastritis effects of an ethanolic extract of the rhizome of Atractylodes macrocephala. J. Ethnopharmacol. 2022, 293, 115251. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Zhao, X.Y.; Lee, J.H.; Lee, J.S.; Keum, Y.S. Ethanol extract of Chaenomeles sinensis inhibits the development of benign prostatic hyperplasia by exhibiting anti-oxidant and anti-inflammatory effects. J. Cancer. Prev. 2022, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Kim, Y.S.; Natarajan, S.B.; Kim, W.S.; Hwang, J.W.; Jeon, N.J.; Jeong, J.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant and anti-inflammatory effects of Chaenomeles sinensis leaf extracts on LPS-stimulated RAW 264.7 cells. Molecules 2016, 21, 422. [Google Scholar] [PubMed]
- Shi, G.; Kong, J.; Wang, Y.; Xuan, Z.; Xu, F. Glycyrrhiza uralensis Fisch. alleviates dextran sulfate sodium-induced colitis in mice through inhibiting of NF-kappaB signaling pathways and modulating intestinal microbiota. J. Ethnopharmacol. 2022, 298, 115640. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Choi, S.J.; Kim, C.R.; Kim, J.K.; Kim, H.K.; Choi, J.H.; Song, S.W.; Kim, C.J.; Park, G.G.; Park, C.S.; et al. Effect of Chaenomeles sinensis extract on choline acetyltransferase activity and trimethyltin-induced learning and memory impairment in mice. Chem. Pharm. Bull. 2015, 6, 1076–1080. [Google Scholar] [CrossRef]
- Li, M.; Ke, J.; Deng, Y.; Chen, C.; Huang, Y.; Bian, Y.; Guo, S.; Wu, Y.; Zhang, H.; Liu, M.; et al. The protective effect of liquiritin in hypoxia/reoxygenation-induced disruption on blood brain barrier. Front. Pharmacol. 2021, 12, 671783. [Google Scholar] [CrossRef]
- Ma, X.; Fang, F.; Song, M.; Ma, S. The effect of isoliquiritigenin on learning and memory impairments induced by high-fat diet via inhibiting TNF-alpha/JNK/IRS signaling. Biochem. Biophys. Res. Commun. 2015, 464, 1090–1095. [Google Scholar]
- Sun, Y.X.; Tang, Y.; Wu, A.L.; Liu, T.; Dai, X.L.; Zheng, Q.S.; Wang, Z.B. Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J. Asian Nat. Prod. Res. 2010, 12, 1051–1060. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, W.; Guo, H.; Zhou, D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav. Brain Res. 2008, 194, 108–113. [Google Scholar]
- Wang, W.; Hu, X.; Zhao, Z.; Liu, P.; Hu, Y.; Zhou, J.; Zhou, D.; Wang, Z.; Guo, D.; Guo, H. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1179–1184. [Google Scholar]
- Ahn, J.; Um, M.; Choi, W.; Kim, S.; Ha, T. Protective effects of Glycyrrhiza uralensis Fisch. on the cognitive deficits caused by beta-amyloid peptide 25–35 in young mice. Biogerontology 2006, 7, 239–247. [Google Scholar]
- Zhou, Y.; Huang, S.; Wu, F.; Zheng, Q.; Zhang, F.; Luo, Y.; Jian, X. Atractylenolide III ameliorates cerebral ischemic injury and neuroinflammation associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial fission in microglia. Phytomedicine 2019, 59, 152922. [Google Scholar] [PubMed]
- Hu, W.X.; Xiang, Q.; Wen, Z.; He, D.; Wu, X.M.; Hu, G.Z. Neuroprotective effect of Atractylodes macrocephalaon polysaccharides in vitro on neuronal apoptosis induced by hypoxia. Mol. Med. Rep. 2014, 9, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, S.; Wu, F.; Zheng, Q.; Zhang, F.; Luo, Y.; Jian, X. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci. Lett. 2021, 759, 136050. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Morinobu, S.; Takei, S.; Fuchikami, M.; Matsuki, A.; Yamawaki, S.; Liberzon, I. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depress. Anxiety 2009, 26, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; López, J.F.; Flagel, S.B.; Vázquez, D.M.; Young, E.A. Young, Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: Relevance to post-traumatic stress disorder. J. Neuroendocrinol. 1999, 11, 11–17. [Google Scholar] [PubMed]
- Pinna, G.; Costa, E.; Guidotti, A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr. Opin. Pharmacol. 2009, 9, 24–30. [Google Scholar] [PubMed]
- Siegmund, A.; Wotjak, C.T. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J. Psychiatr. Res. 2007, 41, 848–860. [Google Scholar]
- Cipriani, A.; Williams, T.; Nikolakopoulou, A.; Salanti, G.; Chaimani, A.; Ipser, J.; Cowen, P.J.; Geddes, J.R.; Stein, D.J. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: A network meta-analysis. Psychol. Med. 2018, 48, 1975–1984. [Google Scholar]
- Kim, H.M.; Zivin, K.; Ganoczy, D.; Pfeiffer, P.; Hoggatt, K.; McCarthy, J.F.; Downing, K.; Valenstein, M. Predictors of alternative antidepressant agent initiation among U. S. veterans diagnosed with depression. Pharmacoepidemiol. Drug Saf. 2010, 19, 1049–1056. [Google Scholar] [CrossRef]
- Drew, M.R.; Huckleberry, K.A. Modulation of aversive memory by adult hippocampal neurogenesis. Neurotherapeutics 2017, 14, 646–661. [Google Scholar] [PubMed]
- Terranova, J.I.; Ogawa, S.K.; Kitamura, T. Adult hippocampal neurogenesis for systems consolidation of memory. Behav. Brain Res. 2019, 372, 112035. [Google Scholar]
- Ghasemi, M.; Navidhamidi, M.; Rezaei, F.; Azizikia, A.; Mehranfard, N. Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms. Cogn. Affect. Behav. Neurosci. 2022, 22, 431–449. [Google Scholar] [PubMed]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Lavado, A.; Lagutin, O.V.; Chow, L.M.; Baker, S.J.; Oliver, G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010, 8, e1000460. [Google Scholar]
- Scharfman, H.E. Functional implications of seizure-induced neurogenesis. Adv. Exp. Med. Biol. 2004, 548, 192–212. [Google Scholar] [PubMed]
- Kasahara, Y.; Nakashima, H.; Nakashima, K. Seizure-induced hilar ectopic granule cells in the adult dentate gyrus. Front. Neurosci. 2023, 17, 1150283. [Google Scholar] [PubMed]
- Cerda, O.; Trimmer, J.S. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci. Lett. 2010, 486, 60–67. [Google Scholar] [CrossRef]
- Kim, J.; Hoffman, D.A. Potassium channels: Newly found players in synaptic plasticity. Neuroscientist 2008, 14, 276–286. [Google Scholar] [CrossRef]
- Ren, C.; Wang, F.; Li, G.; Jiao, Q.; Bai, J.; Yu, D.; Hao, W.; Wang, R.; Cao, J.M. Nerve sprouting suppresses myocardial I(to) and I(K1) channels and increases severity to ventricular fibrillation in rat. Auton Neurosci. 2008, 144, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.P.; Anderson, A.E.; Varga, A.W.; Dineley, K.T.; Cook, R.G.; Pfaffinger, P.J.; Sweatt, J.D. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J. Neurochem. 2000, 75, 2277–2787. [Google Scholar] [PubMed]
- Sweatt, J.D. The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 2001, 76, 1–10. [Google Scholar] [PubMed]
- Bender, R.A.; Lauterborn, J.C.; Gall, C.M.; Cariaga, W.; Baram, T.Z. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur. J. Neurosci. 2001, 13, 679–686. [Google Scholar]
- Nakagawa, S.; Kim, J.E.; Lee, R.; Chen, J.; Fujioka, T.; Malberg, J.; Tsuji, S.; Duman, R.S. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J. Neurosci. 2002, 22, 9868–9876. [Google Scholar] [CrossRef]
- Hetrick, S.E.; Purcell, R.; Garner, B.; Parslow, R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 2010, 7, CD007316. [Google Scholar]
- Thomas, E.; Stein, D.J. Novel pharmacological treatment strategies for posttraumatic stress disorder. Expert. Rev. Clin. Pharmacol. 2017, 10, 167–177. [Google Scholar] [CrossRef]
- Vytal, K.E.; Cornwell, B.R.; Letkiewicz, A.M.; Arkin, N.E.; Grillon, C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Front. Hum. Neurosci. 2013, 7, 93. [Google Scholar] [CrossRef]
- Chai, W.J.; Abd Hamid, A.I.; Abdullah, J.M. Working memory from the psychological and neurosciences perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar]
- Ishikawa, R.; Fukushima, H.; Frankland, P.W.; Kida, S. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. Elife 2016, 5, e17464. [Google Scholar] [CrossRef]
- DeCarolis, N.A.; Eisch, A.J. Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology 2010, 58, 884–893. [Google Scholar] [CrossRef]
- Scharfman, H.E.; Myers, C.E. Corruption of the dentate gyrus by “dominant” granule cells: Implications for dentate gyrus function in health and disease. Neurobiol. Learn Mem. 2016, 129, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hester, M.S.; Danzer, S.C. Hippocampal granule cell pathology in epilepsy—A possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014, 38, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Fournier, N.M.; Caruncho, H.J.; Kalynchuk, L.E. Decreased levels of disrupted-in-schizophrenia 1 (DISC1) are associated with expansion of the dentate granule cell layer in normal and kindled rats. Neurosci. Lett. 2009, 455, 134–139. [Google Scholar] [CrossRef]
- Nawarawong, N.N.; Thompson, K.R.; Guerin, S.P.; Anasooya Shaji, C.; Peng, H.; Nixon, K. Reactive, adult neurogenesis from increased neural progenitor cell proliferation following alcohol dependence in female rats. Front. Neurosci. 2021, 15, 689601. [Google Scholar] [CrossRef]
- Arshad, M.N.; Oppenheimer, S.; Jeong, J.; Buyukdemirtas, B.; Naegele, J.R. Hippocampal transplants of fetal GABAergic progenitors regulate adult neurogenesis in mice with temporal lobe epilepsy. Neurobiol. Dis. 2022, 174, 105879. [Google Scholar]
- Ton, S.T.; Adamczyk, N.S.; Gerling, J.P.; Vaagenes, I.C.; Wu, J.Y.; Hsu, K.; O’Brien, T.E.; Tsai, S.Y.; Kartje, G.L. Dentate gyrus proliferative responses after traumatic brain injury and binge alcohol in adult rats. Neurosci. Insights 2020, 15, 2633105520968904. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, E.L.; Scott, D.; Saatman, K.E. Insulin-like growth factor-1 overexpression increases long-term survival of posttrauma-born hippocampal neurons while inhibiting ectopic migration following traumatic brain injury. Acta Neuropathol. Commun. 2020, 8, 46. [Google Scholar] [CrossRef]
- Hester, M.S.; Tulina, N.; Brown, A.; Barila, G.; Elovitz, M.A. Intrauterine inflammation reduces postnatal neurogenesis in the hippocampal subgranular zone and leads to accumulation of hilar ectopic granule cells. Brain Res. 2018, 1685, 51–59. [Google Scholar] [CrossRef]
- Torres-Reveron, A.; Gray, J.D.; Melton, J.T.; Punsoni, M.; Tabori, N.E.; Ward, M.J.; Frys, K.; Iadecola, C.; Milner, T.A. Early postnatal exposure to methylphenidate alters stress reactivity and increases hippocampal ectopic granule cells in adult rats. Brain Res. Bull. 2009, 78, 175–181. [Google Scholar] [CrossRef]
- Uchida, Y.; Hashimoto, T.; Saito, H.; Takita, K.; Morimoto, Y. Neonatal isoflurane exposure disturbs granule cell migration in the rat dentate gyrus. Biomed. Res. 2022, 43, 1–9. [Google Scholar] [CrossRef]
- Gomes-Leal, W. Adult hippocampal neurogenesis and affective disorders: New neurons for psychic well-being. Front. Neuro. Sci. 2021, 15, 594448. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.B.; Schulkin, J. Hyperexcitability: From normal fear to pathological anxiety and trauma. Front. Syst. Neurosci. 2022, 16, 727054. [Google Scholar] [CrossRef] [PubMed]
- Lothman, E.W.; Stringer, J.L.; Bertram, E.H. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 1992, 7, 301–313. [Google Scholar] [PubMed]
- Jinde, S.; Zsiros, V.; Jiang, Z.; Nakao, K.; Pickel, J.; Kohno, K.; Belforte, J.E.; Nakazawa, K. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron 2012, 76, 1189–1200. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ikeda, Y.; Asada, M.; Inagaki, H.; Kawada, T.; Suzuki, H. Corticosterone facilitates fluoxetine-induced neuronal plasticity in the hippocampus. PLoS ONE 2013, 8, e63662. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, C.; Nivon, L.G.; McEwen, B.S. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 2002, 12, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Netoff, T.; Yuan, L.L. Dendritic mechanisms controlling the threshold and timing requirement of synaptic plasticity. Hippocampus 2011, 21, 288–297. [Google Scholar] [CrossRef]
- Smith, G.D.; Gao, N.J.; Lugo, N. Kv4.2 knockout mice display learning and memory deficits in the Lashley maze. F1000Research 2016, 5, 2456. [Google Scholar] [CrossRef]
- Lugo, J.N.; Brewster, A.L.; Spencer, C.M.; Anderson, A.E. Kv4.2 knockout mice have hippocampal-dependent learning and memory deficits. Learn. Mem. 2012, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Marin-Burgin, A.; Mongiat, L.A.; Pardi, M.B.; Schinder, A.F. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 2012, 335, 1238–1242. [Google Scholar] [CrossRef]
- Tiwari, D.; Schaefer, T.L.; Schroeder-Carter, L.M.; Krzeski, J.C.; Bunk, A.T.; Parkins, E.V.; Snider, A.; Danzer, R.; Williams, M.T.; Vorhees, C.V.; et al. The potassium channel Kv4.2 regulates dendritic spine morphology, electroencephalographic characteristics and seizure susceptibility in mice. Exp. Neurol. 2020, 334, 113437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Rong, X.; Wang, W.; Wang, X. Effects of fluoxetine on protein expression of potassium ion channels in the brain of chronic mild stress rats. Acta Pharm. Sin. B 2015, 5, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Malloy, C.; Tabor, G.T.; Gutzmann, J.J.; Liu, Y.; Abebe, D.; Karlsson, R.M.; Durell, S.; Cameron, H.A.; Hoffman, D.A. Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility. Nat. Commun. 2020, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Martin, K.J.; Arthur, J.S. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem. J. 2014, 458, 469–479. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.R.; Cai, M.; Yang, E.J. Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder. Nutrients 2023, 15, 3815. https://doi.org/10.3390/nu15173815
Park HR, Cai M, Yang EJ. Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder. Nutrients. 2023; 15(17):3815. https://doi.org/10.3390/nu15173815
Chicago/Turabian StylePark, Hee Ra, Mudan Cai, and Eun Jin Yang. 2023. "Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder" Nutrients 15, no. 17: 3815. https://doi.org/10.3390/nu15173815
APA StylePark, H. R., Cai, M., & Yang, E. J. (2023). Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder. Nutrients, 15(17), 3815. https://doi.org/10.3390/nu15173815