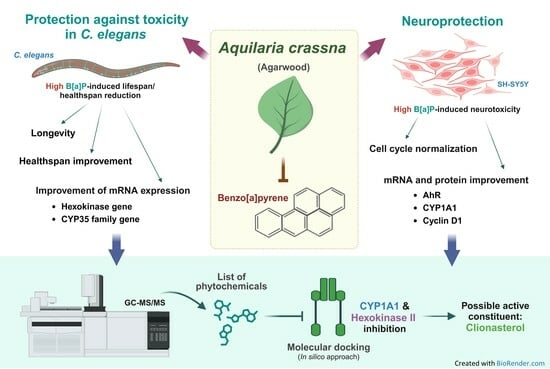
Protective Effect of Aquilaria crassna Leaf Extract against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extraction
2.2. Gas Chromatograph–Mass Spectrometry (GC-MS) Analysis
2.3. Antioxidant Determination
2.3.1. Folin-Ciocalteu Phenol Assay
2.3.2. Assay for Total Flavonoid Content
2.3.3. Radical Scavenging Activity Assays
2.4. Cell Culture
2.5. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Tetrazolium (MTT) Assay
2.6. Cell Cycle Analysis Assay
2.7. Western Blot Analysis
2.8. Cultivation of C. elegans
2.9. C. elegans’ Lifespan Assay
2.10. Measurement of C. elegans’ Body Size and Length
2.11. Quantitative Reverse Transcription PCR (RT-qPCR)
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Constituents of ACEE
3.2. Antioxidant Properties and Total Phenolic and Flavonoid Contents
3.3. Effects of ACEE and B[a]P on Cell Viability
3.4. Effect of ACEE on the Progression of Cell Cycle of SH-SY5Y Co-Cultured with B[a]P
3.5. Effects of ACEE on B[a]P Disturbed Cell Cycle-Associated mRNA and Protein Expression
3.6. Effects of ACEE on B[a]P-Reduced Lifespan of C. elegans
3.7. Effect of ACEE on Toxicity and Metabolism of B[a]P in C. elegans
3.8. Effect of ACEE on mRNA Expression of Cytochrome P450 35 Family (cyp-35) and Hexokinase (hxk) Genes in C. elegans
3.9. The Ability of ACEE-Derived Phytochemical Constituents as Inhibitors of CYP1A1 and Hexokinase II Using an In Silico Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rubis, G.; Paudel, K.R.; Manandhar, B.; Singh, S.K.; Gupta, G.; Malik, R.; Shen, J.; Chami, A.; MacLoughlin, R.; Chellappan, D.K. Agarwood Oil Nanoemulsion Attenuates Cigarette Smoke-Induced Inflammation and Oxidative Stress Markers in BCi-NS1. 1 Airway Epithelial Cells. Nutrients 2023, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Wongwad, E.; Pingyod, C.; Saesong, T.; Waranuch, N.; Wisuitiprot, W.; Sritularak, B.; Temkitthawon, P.; Ingkaninan, K. Assessment of the bioactive components, antioxidant, antiglycation and anti-inflammatory properties of Aquilaria crassna Pierre ex Lecomte leaves. Ind. Crops Prod. 2019, 138, 111448. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Sornkaew, N.; Warayanon, W.; Rangsinth, P.; Sillapachaiyaporn, C.; Vongthip, W.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Aquilaria crassna leaf extract ameliorates glucose-induced neurotoxicity in vitro and improves lifespan in caenorhabditis elegans. Nutrients 2022, 14, 3668. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Merelli, A.; Czornyj, L.; Lazarowski, A. Erythropoietin: A neuroprotective agent in cerebral hypoxia, neurodegeneration, and epilepsy. Curr. Pharm. Des. 2013, 19, 6791–6801. [Google Scholar] [CrossRef]
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110, 451–488. [Google Scholar]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo [a] pyrene—Environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Hardonnière, K.; Saunier, E.; Lemarié, A.; Fernier, M.; Gallais, I.; Héliès-Toussaint, C.; Mograbi, B.; Antonio, S.; Bénit, P.; Rustin, P. The environmental carcinogen benzo [a] pyrene induces a Warburg-like metabolic reprogramming dependent on NHE1 and associated with cell survival. Sci. Rep. 2016, 6, 30776. [Google Scholar] [CrossRef]
- Tancell, P.; Rhead, M.; Trier, C.; Bell, M.; Fussey, D. The sources of benzo [a] pyrene in diesel exhaust emissions. Sci. Total Environ 1995, 162, 179–186. [Google Scholar] [CrossRef]
- Bukowska, B.; Sicińska, P. Influence of benzo (a) pyrene on different epigenetic processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef]
- Knafla, A.; Phillipps, K.; Brecher, R.; Petrovic, S.; Richardson, M. Development of a dermal cancer slope factor for benzo [a] pyrene. Regul. Toxicol. Pharmacol. 2006, 45, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Patel, B.; Patri, M. Neurotoxic effect of benzo [a] pyrene and its possible association with 6-hydroxydopamine induced neurobehavioral changes during early adolescence period in rats. J. Toxicol. 2016, 2016, 8606410. [Google Scholar] [CrossRef] [PubMed]
- Jorge, B.C.; Reis, A.C.C.; Stein, J.; da Silva Balin, P.; Sterde, E.T.; Barbosa, M.G.; de Aquino, A.M.; Kassuya, C.A.L.; Arena, A.C. Parental exposure to benzo (a) pyrene in the peripubertal period impacts reproductive aspects of the F1 generation in rats. Reprod. Toxicol. 2021, 100, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.R.; Das, S.K.; Ramesh, A.; Shockley, D.C.; Mukherjee, S. Benzo (a) pyrene–induced acute neurotoxicity in the F–344 rat: Role of oxidative stress. J. Appl. Toxicol. Int. J. 2006, 26, 427–438. [Google Scholar] [CrossRef]
- Menzel, R.; Bogaert, T.; Achazi, R. A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Arch. Biochem. Biophys. 2001, 395, 158–168. [Google Scholar] [CrossRef]
- Menzel, R.; Rödel, M.; Kulas, J.; Steinberg, C.E. CYP35: Xenobiotically induced gene expression in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 2005, 438, 93–102. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Tencomnao, T. Citrus hystrix extracts protect human neuronal cells against high glucose-induced senescence. Pharmaceuticals 2020, 13, 283. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Meemon, K.; Sobhon, P.; Tencomnao, T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complement. Altern. Med. 2017, 17, 551. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Hasler, A.; Sticher, O.; Meier, B. Identification and determination of the flavonoids from Ginkgo biloba by high-performance liquid chromatography. J. Chromatogr. A 1992, 605, 41–48. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Williams, D.E. Structure of 2,2-Diphenyl-1-picrylhydrazyl Free Radical1. J. Am. Chem. Soc. 1966, 88, 5665–5666. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. BBA—Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Septisetyani, E.P.; Ningrum, R.A.; Romadhani, Y.; Wisnuwardhani, P.H.; Santoso, A. Optimization of sodium dodecyl sulphate as a formazan solvent and comparison of 3-(4,-5-dimethylthiazo-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay with wst-1 assay in mcf-7 cells. Indones. J. Pharm. 2014, 25, 245. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Rakkhitawatthana, V.; Tencomnao, T. Effect of Gloriosa superba and Catharanthus roseus extracts on IFN-γ-induced keratin 17 expression in HaCaT human keratinocytes. eCAM 2014, 2014, 249367. [Google Scholar] [PubMed]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef]
- Kaewjanthong, P.; Sooksai, S.; Sasano, H.; Hutvagner, G.; Bajan, S.; McGowan, E.; Boonyaratanakornkit, V. Cell-penetrating peptides containing the progesterone receptor polyproline domain inhibits EGF signaling and cell proliferation in lung cancer cells. PLoS ONE 2022, 17, e0264717. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Prasanth, M.I.; Verma, K.; Prasansuklab, A.; Tencomnao, T. Hibiscus sabdariffa Extract Protects HaCaT Cells against Phenanthrene-Induced Toxicity through the Regulation of Constitutive Androstane Receptor/Pregnane X Receptor Pathway. Nutrients 2022, 14, 3829. [Google Scholar] [CrossRef]
- Gu, Q.L.; Zhang, Y.; Fu, X.M.; Lu, Z.L.; Yu, Y.; Chen, G.; Ma, R.; Kou, W.; Lan, Y.M. Toxicity and metabolism of 3-bromopyruvate in Caenorhabditis elegans. J. Zhejiang Univ. Sci. B 2020, 21, 77–86. [Google Scholar] [CrossRef]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef]
- Lin, H.; Zeng, J.; Xie, R.; Schulz, M.J.; Tedesco, R.; Qu, J.; Erhard, K.F.; Mack, J.F.; Raha, K.; Rendina, A.R.; et al. Discovery of a Novel 2,6-Disubstituted Glucosamine Series of Potent and Selective Hexokinase 2 Inhibitors. ACS Med. Chem. Lett. 2016, 7, 217–222. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Prasansuklab, A.; Tencomnao, T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients 2021, 13, 2368. [Google Scholar] [CrossRef] [PubMed]
- Chaikhong, K.; Chumpolphant, S.; Rangsinth, P.; Sillapachaiyaporn, C.; Chuchawankul, S.; Tencomnao, T.; Prasansuklab, A. Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants 2023, 12, 65. [Google Scholar] [CrossRef]
- Takahashi, E.; Fujita, K.-I.; Kamataki, T.; Arimoto-Kobayashi, S.; Okamoto, K.; Negishi, T. Inhibition of human cytochrome P450 1B1, 1A1 and 1A2 by antigenotoxic compounds, purpurin and alizarin. Mutat. Res. Fundam. Mol. 2002, 508, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, J.M. Metrizamide inhibits human brain hexokinase. Neurology 1982, 32, 884. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The potential of lonidamine in combination with chemotherapy and physical therapy in cancer treatment. Cancers 2020, 12, 3332. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Guo, L.; Nancolas, B.; Nelson, D.S.; Shestov, A.A.; Lee, S.-C.; Roman, J.; Zhou, R.; Leeper, D.B.; Halestrap, A.P. Mechanism of antineoplastic activity of lonidamine. BBA-Rev. Cancer 2016, 1866, 151–162. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Fromenty, B. Mitochondrial dysfunction induced by xenobiotics: Involvement in steatosis and steatohepatitis. In Mitochondria in Obesity and Type 2 Diabetes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–364. [Google Scholar]
- Gao, D.; Wu, M.; Wang, C.; Wang, Y.; Zuo, Z. Chronic exposure to low benzo [a] pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 167, 200–208. [Google Scholar] [CrossRef]
- Manzella, C.; Singhal, M.; Alrefai, W.A.; Saksena, S.; Dudeja, P.K.; Gill, R.K. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci. Rep. 2018, 8, 6103. [Google Scholar] [CrossRef]
- Kabadi, S.V.; Stoica, B.A.; Loane, D.J.; Byrnes, K.R.; Hanscom, M.; Cabatbat, R.M.; Tan, M.T.; Faden, A.I. Cyclin D1 gene ablation confers neuroprotection in traumatic brain injury. J. Neurotrauma 2012, 29, 813–827. [Google Scholar] [CrossRef]
- Abbass, M.; Chen, Y.; Arlt, V.M.; Stürzenbaum, S.R. Benzo [a] pyrene and Caenorhabditis elegans: Defining the genotoxic potential in an organism lacking the classical CYP1A1 pathway. Arch. Toxicol. 2021, 95, 1055–1069. [Google Scholar] [CrossRef]
- Aarnio, V.; Lehtonen, M.; Storvik, M.; Callaway, J.C.; Lakso, M.; Wong, G. Caenorhabditis elegans mutants predict regulation of fatty acids and endocannabinoids by the CYP-35A gene family. Front. Pharmacol. 2011, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Imanikia, S.; Hylands, P.; Stürzenbaum, S.R. The double mutation of cytochrome P450’s and fatty acid desaturases affect lipid regulation and longevity in C. elegans. Biochem. Biophys. 2015, 2, 172–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X.; Ding, Y.; Wang, H.; Wu, X.; Liang, B. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 2013, 14, 164. [Google Scholar] [CrossRef]
- Thies, J.L.; Willicott, K.; Craig, M.L.; Greene, M.R.; DuGay, C.N.; Caldwell, G.A.; Caldwell, K.A. Xanthine Dehydrogenase Is a Modulator of Dopaminergic Neurodegeneration in Response to Bacterial Metabolite Exposure in C. elegans. Cells 2023, 12, 1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, X. Virtual Screening and Biological Activity Evaluation of New Potent Inhibitors Targeting Hexokinase-II. Molecules 2022, 27, 7555. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E. Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J. Exp. Biol. 2003, 206, 2049–2057. [Google Scholar] [CrossRef]
- de Meis, L.; Grieco, M.A.B.; Galina, A. Reversal of oxidative phosphorylation in submitochondrial particles using glucose 6-phosphate and hexokinase as an ATP regenerating system. FEBS Lett. 1992, 308, 197–201. [Google Scholar] [CrossRef]
- Luecha, P.; Umehara, K.; Miyase, T.; Noguchi, H. Antiestrogenic constituents of the Thai medicinal plants Capparis flavicans and Vitex glabrata. J. Nat. Prod. 2009, 72, 1954–1959. [Google Scholar] [CrossRef]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, B.B.; Hasdemir, B.; Yusufoglu, A.; Yanardag, R. Some monohydroxy tetradecanoic acid isomers as novel urease and elastase inhibitors and as new antioxidants. Appl. Biochem. Biotechnol. 2014, 172, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.A.; Kim, S.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and isolated 6-hydroxy-4, 4, 7a-trimethyl-5, 6, 7, 7a-tetrahydrobenzofuran-2 (4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Raman, B.V.; Samuel, L.; Saradhi, M.P.; Rao, B.N.; Krishna, N.; Sudhakar, M.; Radhakrishnan, T. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012, 5, 99–106. [Google Scholar]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Obuotor, E.M.; Agbedahunsi, J.M.; Adesanya, S.A. Anticholinesterase constituents from the leaves of Spondias mombin L.(Anacardiaceae). Biol. Targets Ther. 2017, 11, 107–114. [Google Scholar] [CrossRef]
- Çakmak, Y.S.; Aktumsek, A.; Duran, A. Studies on antioxidant activity, volatile compound and fatty acid composition of different parts of Glycyrrhiza echinata L. EXCLI J. 2012, 11, 178. [Google Scholar]
- Tyagi, T.; Agarwal, M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J. Pharmacogn. Phytochem. 2017, 6, 195–206. [Google Scholar]
- Choi, J.; Shin, K.-M.; Park, H.-J.; Jung, H.-J.; Kim, H.J.; Lee, Y.S.; Rew, J.-H.; Lee, K.-T. Anti-inflammatory and antinociceptive effects of sinapyl alcohol and its glucoside syringin. Planta Med. 2004, 70, 1027–1032. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Lee, J.H.; Kim, J.-E.; Ha, J.; Kang, I. Oleamide suppresses lipopolysaccharide-induced expression of iNOS and COX-2 through inhibition of NF-κB activation in BV2 murine microglial cells. Neurosci. Lett. 2010, 474, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef]
- Heo, H.-J.; Park, Y.-J.; Suh, Y.-M.; Choi, S.-J.; Kim, M.-J.; Cho, H.-Y.; Chang, Y.-J.; Hong, B.; Kim, H.-K.; Kim, E. Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci. Biotechnol. Biochem. 2003, 67, 1284–1291. [Google Scholar] [CrossRef]
- Saint–Leger, D.; Bague, A.; Lefebvre, E.; Cohen, E.; Chivot, M. A possible role for squalene in the pathogenesis of acne. II. In vivo study of squalene oxides in skin surface and intra–comedonal lipids of acne patients. Br. J. Dermatol. 1986, 114, 543–552. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Bioactivity of vitamin E. Nutr. Res. Rev. 2006, 19, 174–186. [Google Scholar] [CrossRef]
- Cerqueira, F.; Watanadilok, R.; Sonchaeng, P.; Kijjoa, A.; Pinto, M.; van Ufford, H.Q.; Kroes, B.; Beukelman, C.; Nascimento, M.S.J. Clionasterol: A potent inhibitor of complement component C1. Planta Med. 2003, 69, 174–176. [Google Scholar] [CrossRef]
- Liyanage, N.; Nagahawatta, D.; Jayawardena, T.U.; Jayawardhana, H.; Lee, H.-G.; Kim, Y.-S.; Jeon, Y.-J. Clionasterol-Rich Fraction of Caulerpa racemosa against Particulate Matter-Induced Skin Damage via Inhibition of Oxidative Stress and Apoptosis-Related Signaling Pathway. Antioxidants 2022, 11, 1941. [Google Scholar] [CrossRef]
- Niu, X.; Yao, H.; Li, W.; Mu, Q.; Li, H.; Hu, H.; Li, Y.; Huang, H. δ-Amyrone, a specific inhibitor of cyclooxygenase-2, exhibits anti-inflammatory effects in vitro and in vivo of mice. Int. Immunopharmacol. 2014, 21, 112–118. [Google Scholar] [CrossRef]
- da Silva, K.A.S.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α, β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. PAIN 2011, 152, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Bahena, S.M.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement. Altern. Med. 2016, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K.; Yu, S.; Yamanouchi, S.; Takido, M.; Akihisa, T.; Tamura, T. Some lupane-type triterpenes inhibit tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Phytomedicine 1995, 1, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Sukari, M.A.; Ismail, N.; Ismail, I.S.; Abdul, A.B.; Abu Bakar, M.F.; Kifli, N.; Ee, G.C. Phytochemicals from Mangifera pajang Kosterm and their biological activities. BMC Complement. Altern. Med. 2015, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Suwito, H.; Heffen, W.L.; Cahyana, H.; Suwarso, W.P. Isolation, transformation, anticancer, and apoptosis activity of lupeyl acetate from Artocarpus integra. AIP Conf. Proc. 2016, 1718, 080004. [Google Scholar]
- Odeh, I.C.; Tor-Anyiin, T.A.; Igoli, J.O.; Anyam, J.V. In vitro antimicrobial properties of friedelan-3-one from Pterocarpus santalinoides L’Herit, ex Dc. Afr. J. Biotechnol. 2016, 15, 531–538. [Google Scholar]
- Lin, C.-H.; Kuo, Y.-H.; Shih, C.-C. Eburicoic acid, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects in palmitate-treated C2C12 myotubes and in high-fat diet-fed mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, P.; Yang, J.; Huang, J.; Xu, P.; Hu, M.; Zhang, C.; Wang, B.; Peng, W. Integrated network pharmacology analysis and serum metabolomics to reveal the cognitive improvement effect of Bushen Tiansui formula on Alzheimer’s disease. J. Ethnopharmacol. 2020, 249, 112371. [Google Scholar] [CrossRef]












| Primer Sequences for SH-SY5Y | |||
|---|---|---|---|
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Ref. |
| ACTB | GGCATCCTCACCCTGAAGTA | AGCCTGGATAGCAACGTACA | * |
| CCND1 | ATGTTCGTGGCCTCTAAGATGA | CAGGTTCCACTTGAGCTTGTTC | [27] |
| AHR | GTCGTCTAAGGTGTCTGCTGGA | CGCAAACAAAGCCAACTGAGGTG | [28] |
| CYP1A1 | GATTGAGCACTGTCAGGAGAAGC | ATGAGGCTCCAGGAGATAGCAG | |
| Primer Sequences for C. elegans | |||
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) | Ref. |
| act-1 | AGACAATGGATCCGGAATGT | CATCCCAGTTGGTGACGATA | [3] |
| cyp-35A2 | GCATCCATTCTTAACGTCAGCTTC | ATCTTCGGTAACCTTCTCCTTCG | [29] |
| cyp-35A4 | ACCAAATCAAGTCTGGGAGGTA | TCTTACTGACCGTGCTTCAACTC | |
| cyp-35A5 | CATCTTCACCTTGTGGGTTGG | TTAGAAATATGGGCTTCGGGAAGG | |
| cyp-35B3 | GTGATTATGAAACGTCGCAAGAAG | GCGGATGCTGTAAATGGAAAGAC | |
| hxk-1 | GGAGAGTGTGCCCGAGTTGT | ATGCTCTCTGAAGATGGATCTGG | |
| hxk-2 | GCTCTTTAATGGAATTGGCTCG | CGCAATCGTTTCGAGAGTCA | |
| hxk-3 | CAAAGCAGTGATGAACGACACA | GACACAATTTGATCGGGAAGTCG | |
| cyp-35A1 | GTCCACGCTTGATCTGTTCC | CTCCAGTGACTACCGTTCGT | * |
| cyp-35A3 | GCAGATAAACTACACGCCCC | TGTGCTACAGTGACTCCGTT | |
| cyp-35B2 | CTGTGAACGCTGAGAATCCG | CGTGAGCCATTTTCCGTGAT | |
| cyp-35C1 | TAACCGGCCAAGAAACAACG | TGAGGATGGATGCATGTCGT | |
| Compound | RT | Area (%) | MF | MW |
|---|---|---|---|---|
| Glyceraldehyde | 2.871 | 0.62 | C3H6O3 | 90 |
| 3′Hydroxyacetophenone | 17.056 | 0.28 | C8H8O2 | 136 |
| 4-Hydroxybenzoic acid | 18.612 | 0.51 | C7H6O3 | 138 |
| (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol | 23.897 | 0.07 | C10H12O3 | 180 |
| Tetradecanoic acid | 24.359 | 0.07 | C14H28O2 | 228 |
| 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | 24.518 | 0.19 | C11H16O3 | 196 |
| Neophytadiene | 26.03 | 8.53 | C20H38 | 278 |
| 3,7,11,15-Tetramethylhexadec-2-ene | 26.172 | 0.74 | C20H40 | 280 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) | 26.890 | 2.43 | C20H40O | 296 |
| n-Hexadecanoic acid | 28.430 | 1.01 | C16H32O2 | 256 |
| trans-Sinapyl alcohol | 29.001 | 0.11 | C11H14O4 | 210 |
| Hexadecanoic acid, ethyl ester | 29.070 | 0.08 | C18H36O2 | 284 |
| Linoleic acid ethyl ester | 32.253 | 0.05 | C20H36O2 | 308 |
| Hexadecanamide | 32.418 | 0.1 | C16H33NO | 255 |
| 9-Octadecenamide, (Z)- | 35.489 | 1.58 | C18H35NO | 281 |
| Squalene | 42.527 | 23.1 | C30H50 | 410 |
| Vitamin E | 46.533 | 10.00 | C29H50O2 | 430 |
| Clionasterol | 48.825 | 5.85 | C29H50O | 414 |
| β-Amyrone | 48.968 | 5.85 | C30H48O | 424 |
| β-Amyrin | 49.285 | 4.05 | C30H50O | 426 |
| Lupenone | 49.603 | 6.6 | C30H48O | 424 |
| Friedelan-3-one | 51.653 | 12.76 | C30H50O | 426 |
| Acetyleburicoic acid | 51.979 | 6.05 | C33H52O4 | 512 |
| 0.1% DMSO (%) | 40 µM B[a]P (%) | 40 µM B[a]P + 5 µg/mL ACEE (%) | |
|---|---|---|---|
| G0/G1 | 49.84 ± 3.02 | 40.83 ± 1.05 * | 50.49 ± 0.95 # |
| S | 16.32 ± 1.26 | 12.55 ± 1.62 | 16.65 ± 1.71 |
| G2M | 16.76 ± 8.58 | 17.33 ± 1.89 | 15.29 ± 1.05 |
| Group | Mean Lifespan | p-Value (vs. 40 µM B[a]P) | Number of Worms | |
|---|---|---|---|---|
| Day ± SEM | % Increase (vs. 40 µM B[a]P) | |||
| 0.1% DMSO | 15.40 ± 0.26 | 13.23 | 0.0169 | 154 |
| 40 µM B[a]P | 13.60 ± 0.35 | - | - | 145 |
| 40 µM B[a]P + | ||||
| 5 µg/mL ACEE | 15.42 ± 0.26 | 13.38 | 0.0071 | 173 |
| No. | Compound | Binding Energy (kcal/mol) | Inhibition Constant | Amino Acid Interaction | ||
|---|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Electrostatic Bond | ||||
| alpha-naphthoflavone (Original ligand) | −10.16 | 35.57 nM | ||||
| Alizarin (Positive control) | −8.56 | 535.22 nM | SER116 ASN255 | ILE115 PHE224 (3) | ||
| Purpurin (Positive control) | −8.97 | 267.12 nM | SER116 ASN255 (2) LEU312 | ILE115 PHE224 (3) PHE258 (3) LEU312 | ||
| 1 | Neophytadiene | −7.83 | 1.83 µM | ILE115 PHE123 PHE224 (5) LEU254 PHE258 (3) LEU312 (2) ALA317 (2) PHE319 (2) LEU496 | ||
| 2 | Vitamin E | −10.0 | 46.57 nM | SER116 GLY316 | ILE115 (2) PHE224 (4) PHE258 (3) LEU312 ALA317 (2) PHE319 VAL382 ILE386 CYS457 LEU496 (2) | |
| 3 | β-amyrone | −10.12 | 38.35 nM | ILE115 (2) PHE123 (3) PHE224 (6) PHE258 (2) LEU312 ALA317 (2) PHE319 ILE386 | ||
| 4 | β-amyrin | −1.17 | 137.92 mM | ILE115 PHE123 (2) PHE224 (2) ALA317 (3) VAL382 (2) ILE386 (3) CYS457 (3) LEU496 | ||
| 5 | Friedelan-3-one | −7.52 | 3.10 µM | ASP313 | PHE224 PHE258 (2) PHE319 | |
| 6 | Clionasterol | −10.45 | 22.02 nM | ILE115 PHE123 PHE224 (3) ALA317 (3) VAL382 (2) ILE386 (2) LEU496 (3) | ||
| 7 | Squalene | −8.92 | 287.33 nM | ILE115 PHE123 PHE224 (3) PHE258 (2) LEU312 (2) ALA317 (4) PHE319 ILE386 CYS457 (3) ILE458 LEU496 | ||
| 8 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) | −6.81 | 10.21 µM | PHE258 GLY316 | ILE115 (2) PHE123 (2) PHE224 (5) LEU254 PHE258 (2) LEU312 (2) ALA317 (2) LEU496 | |
| 9 | n-Hexadecanoic acid (Palmitic acid) | −5.28 | 134.07 µM | SER122 | ILE115 PHE123 (2) PHE224 (3) PHE258 LEU312 ALA317 (2) PHE319 | |
| 10 | 9-Octadecenamide, (Z)- | −6.30 | 24.26 µM | ASP320 | ILE115 PHE224 (6) PHE258 ALA317 (2) LEU254 LEU312 PHE319 | |
| 11 | Lupenone | −6.41 | 20.16 µM | ILE115 PHE123 PHE224 (2) ALA317 (5) PHE319 LEU496 | ||
| No. | Compound | Binding Energy (kcal/mol) | Inhibition Constant | Amino Acid Interaction | ||
|---|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Electrostatic Bond | ||||
| 2-deoxy-2-{[(2E)-3-(3,4-dichlorophenyl)prop-2-enoyl]amino}7-alpha-D-glucopyranose (Original ligand) | −6.52 | 16.65 µM | ||||
| Metrizamide (Positive control) | −6.53 | 16.40 µM | LYS62 CYS158 GLU260 ASN287 GLU294 (2) | PRO157 (2) | GLU260 | |
| Lonidamine (Positive control) | −4.58 | 441.25 µM | CYS158 GLU260 | PHE154 PRO157 (4) CYS158 (2) | ||
| 1 | Neophytadiene | −4.48 | 515.98 µM | PHE154 PRO157 CYS158 (2) VAL206 ILE229 | ||
| 2 | Vitamin E | −6.43 | 19.36 µM | CYS158 | PRO157 (4) CYS158 ILE229 (2) MET300 | GLU260 |
| 3 | β-amyrone | −6.51 | 16.95 µM | PRO157 (2) CYS158 VAL206 | ||
| 4 | β-amyrin | −7.46 | 3.42 µM | ASP209 ASN235 | PRO157 ILE229 | |
| 5 | Friedelan-3-one | −4.96 | 231.14 µM | ASN208 ASN235 | ||
| 6 | Clionasterol | −6.64 | 13.56 µM | LYS62 LEU64 (3) PRO157 (3) CYS158 ALA263 LYS290 LYS290 | ||
| 7 | Squalene | −4.20 | 840.58 µM | LYS62 (2) LEU64 (2) PHE154 PRO157 (4) CYS158 (2) VAL206 ALA263 (2) | ||
| 8 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) | −3.58 | 2.38 mM | GLU260 | LYS62 LEU64 PRO157 (4) ALA263 | |
| 9 | n-Hexadecanoic acid (Palmitic acid) | −2.31 | 20.31 mM | THR232 | ILE229 | |
| 10 | 9-Octadecenamide, (Z)- | −3.86 | 1.49 mM | ASN208 ASN235 GLU260 | LYS62 (2) LEU64 LEU64 PRO157 (3) CYS158 ALA263 | |
| 11 | Lupenone | −7.05 | 6.85 µM | ASN208 THR210 ASN235 | ILE229 MET300 (2) TYR301 | |
| Compound | Bioactivity |
|---|---|
| 4-Hydroxybenzoic acid | Anti-inflammatory activities [50] Antioxidant activities [51,52,53] |
| Tetradecanoic acid | Some monohydroxy tetradecanoic acid isomers provide urease and elastase inhibitors, and antioxidants [54] |
| 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | Anti-inflammatory activity [55] |
| Neophytadiene | Antimicrobial, antifungal, anti-inflammatory and antioxidant activities [56] |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) | Antioxidant and neuroprotective effects [57,58] Anticholinesterase activity [59] |
| n-Hexadecanoic acid (Palmitic acid) | Anti-inflammatory activities Antioxidant activities [60,61] |
| trans-Sinapyl alcohol | Anti-inflammatory and antinociceptive activities [62] |
| Hexadecanoic acid, ethyl ester (Ethyl palmitate) | Anti-inflammatory activities [63] |
| Linoleic acid ethyl ester | Anti-inflammatory activities [64] |
| 9-Octadecenamide, (Z)-(Oleamide) | Anti-inflammatory activities [65] Chemoprotective agent against Alzheimer’ s disease [66,67] |
| Squalene | Antioxidant activity [68] |
| Vitamin E | Antioxidant activity [63,69] |
| Clionasterol | Anticomplementary effect [70] Inhibition of particulate matter-induced oxidative stress and apoptosis in skin [71] |
| β-Amyrone | Anti-inflammatory activity [72] |
| β-Amyrin | Anti-inflammatory activity [73] |
| Lupenone | Anti-inflammatory activity [74,75] Anticancer [76,77] |
| Friedelan-3-one | Antimicrobial properties [78] |
| Acetyleburicoic acid | Antidiabetic and antihyperlipidemic effects [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarachotanant, N.; Rangsinth, P.; Warayanon, W.; Leung, G.P.-H.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Protective Effect of Aquilaria crassna Leaf Extract against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol. Nutrients 2023, 15, 3985. https://doi.org/10.3390/nu15183985
Pattarachotanant N, Rangsinth P, Warayanon W, Leung GP-H, Chuchawankul S, Prasansuklab A, Tencomnao T. Protective Effect of Aquilaria crassna Leaf Extract against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol. Nutrients. 2023; 15(18):3985. https://doi.org/10.3390/nu15183985
Chicago/Turabian StylePattarachotanant, Nattaporn, Panthakarn Rangsinth, Watis Warayanon, George Pak-Heng Leung, Siriporn Chuchawankul, Anchalee Prasansuklab, and Tewin Tencomnao. 2023. "Protective Effect of Aquilaria crassna Leaf Extract against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol" Nutrients 15, no. 18: 3985. https://doi.org/10.3390/nu15183985
APA StylePattarachotanant, N., Rangsinth, P., Warayanon, W., Leung, G. P. -H., Chuchawankul, S., Prasansuklab, A., & Tencomnao, T. (2023). Protective Effect of Aquilaria crassna Leaf Extract against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol. Nutrients, 15(18), 3985. https://doi.org/10.3390/nu15183985