Natural Activators of Autophagy Reduce Oxidative Stress and Muscle Injury Biomarkers in Endurance Athletes: A Pilot Study
Abstract
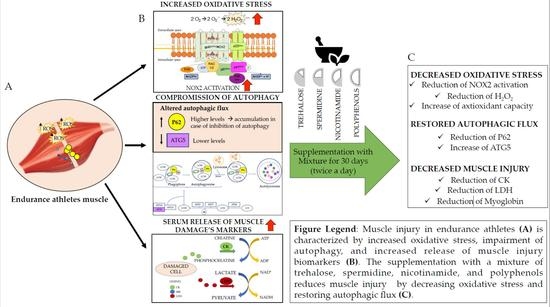
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Mixture Composition
2.4. Blood Sampling and Preparations
2.5. Evaluation of Oxidative Stress
2.6. Evaluation of Muscle Damage
2.7. Evaluation of Autophagy Biomarkers
2.7.1. Plasma ATG5 Detection
2.7.2. Plasma p62 Detection
2.8. Sample Size Calculation
2.9. Statistical Analyses
3. Results
3.1. Muscle Damage
3.2. Oxidative Stress
3.3. Autophagy
3.4. Linear Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, R.; Ribeiro, F.; Alberto Duarte, J.; Cabri, J.M.H. Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: Systematic review and meta-analysis. Phys. Ther. Sport 2012, 13, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between oxidant and antioxidant systems: Short- and Long-Term implications for athletes’ health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [Green Version]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’amico, A.; Cavarretta, E.; Fossati, C.; Borrione, P.; Pigozzi, F.; Frati, G.; Sciarretta, S.; Costa, V.; De Grandis, F.; Nigro, A.; et al. Platelet Activation Favours NOX2-Mediated Muscle Damage in Elite Athletes: The Role of Cocoa-Derived Polyphenols. Nutrients 2022, 14, 1558. [Google Scholar] [CrossRef]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Zhou, X.J.; Zhang, H. Exploring the role of autophagy-related gene 5 (ATG5ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front. Immunol. 2018, 9, 2334. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Chapter 12 Monitoring Autophagic Degradation of p62/SQSTM1. Methods Enzymol. 2009, 451, 181–197. [Google Scholar]
- Sciarretta, S.; Forte, M.; Castoldi, F.; Frati, G.; Versaci, F.; Sadoshima, J.; Kroemer, G.; Maiuri, M.C. Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc. Res. 2021, 117, 1434–1449. [Google Scholar] [CrossRef] [PubMed]
- Frati, G.; Vecchione, C.; Sciarretta, S. Novel beneficial cardiovascular effects of natural activators of autophagy. Circ. Res. 2018, 123, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Cho, E.D.; Ahn, W.J.; Lee, K.W.; Lee, S.J.; Lee, H.J. Is trehalose an autophagic inducer? Unraveling the roles of non-reducing disaccharides on autophagic flux and alpha-synuclein aggregation. Cell Death Dis. 2017, 8, e3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciarretta, S.; Yee, D.; Nagarajan, N.; Bianchi, F.; Saito, T.; Valenti, V.; Tong, M.; Del Re, D.P.; Vecchione, C.; Schirone, L.; et al. Trehalose-Induced Activation of Autophagy Improves Cardiac Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Korolenko, T.A.; Ovsyukova, M.V.; Bgatova, N.P.; Ivanov, I.D.; Makarova, S.I.; Vavilin, V.A.; Popov, A.V.; Yuzhik, E.I.; Koldysheva, E.V.; Korolenko, E.C.; et al. Trehalose Activates Hepatic and Myocardial Autophagy and Has Anti-Inflammatory Effects in db/db Diabetic Mice. Life 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zheng, C.; Cao, J.; Cao, G.; Shou, P.; Lin, L.; Velletri, T.; Jiang, M.; Chen, Q.; Han, Y.; et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef]
- Gupta, V.K.; Scheunemann, L.; Eisenberg, T.; Mertel, S.; Bhukel, A.; Koemans, T.S.; Kramer, J.M.; Liu, K.S.Y.; Schroeder, S.; Stunnenberg, H.G.; et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 2013, 16, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.P.; Baracco, E.E.; Levesque, S.; Castoldi, F.; Jacquelot, N.; Yamazaki, T.; et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016, 30, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wang, J.; Jiang, H.; Liu, X.; Sun, X.; Chen, Y.; Hu, C.; Wang, Z.; Han, T.; Sun, C.; et al. The association of dietary spermidine with all-cause mortality and CVD mortality: The U.S. National Health and Nutrition Examination Survey, 2003 to 2014. Front. Public Health 2022, 10, 949170. [Google Scholar] [CrossRef]
- Omar, E.; Omar, R.; Shoela, M.; El Sayed, N. A study of the cardioprotective effect of spermidine: A novel inducer of autophagy. Chin. J. Physiol. 2021, 64, 281–288. [Google Scholar] [CrossRef]
- Michiels, C.F.; Kurdi, A.; Timmermans, J.P.; De Meyer, G.R.Y.; Martinet, W. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis 2016, 251, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T.; Hwang, E.S. Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell 2009, 8, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Bianchi, F.; Cotugno, M.; Marchitti, S.; De Falco, E.; Raffa, S.; Stanzione, R.; Di Nonno, F.; Chimenti, I.; Palmerio, S.; et al. Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy 2020, 16, 1468–1481. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Schiavon, S.; Cammisotto, V.; Cotugno, M.; Forte, M.; Valenti, V.; Marchitti, S.; Vecchio, D.; Biondi Zoccai, G.; et al. Beneficial effects of a combination of natural product activators of autophagy on endothelial cells and platelets. Br. J. Pharmacol. 2021, 178, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Mannucci, A.; Barygina, V.; Mascherini, G.; Emmi, G.; Silvestri, E.; Wright, D.; Taddei, N.; Galanti, G.; Fiorillo, C. Redox status alterations during the competitive season in élite soccer players: Focus on peripheral leukocyte-derived ROS. Intern. Emerg. Med. 2017, 12, 777–788. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Sanguigni, V.; Violi, F.; Carnevale, R. A novel role of MMP2 in regulating platelet NOX2 activation. Free Radic. Biol. Med. 2020, 152, 355–362. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Pignatelli, P.; Bartimoccia, S.; Stefanini, L.; Basili, S.; Novo, M.; D’Amico, A.; Cammisotto, V.; Pastori, D.; et al. Blood hydrogen peroxide break-down activity in healthy subjects and in patients at risk of cardiovascular events. Atherosclerosis 2018, 274, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sport. Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark chocolate intake positively modulates redox status and markers of muscular damage in elite football athletes: A randomized controlled study. Oxid. Med. Cell. Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef]
- Mizushima, N.; Yamamoto, A.; Matsui, M.; Yoshimori, T.; Ohsumi, Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol. Biol. Cell 2004, 15, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.T.; Siu, P.M. Autophagic cellular responses to physical exercise in skeletal muscle. Sport. Med. 2014, 44, 625–640. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sumpter, R., Jr.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012, 8, 1548–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.A.; Kim, Y.S.; Song, W. Autophagic response to a single bout of moderate exercise in murine skeletal muscle. J. Physiol. Biochem. 2012, 68, 229–235. [Google Scholar] [CrossRef]
- Rodney, G.G.; Pal, R.; Abo-Zahrah, R. Redox regulation of autophagy in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Wadazumi, T.; Watanabe, K.; Watanabe, H.; Yokoyama, H.; Hongu, N.; Arai, N. Effects of a single ingestion of trehalose during prolonged exercise. Sports 2019, 7, 100. [Google Scholar] [CrossRef] [Green Version]
- Hamada, N.; Wadazumi, T.; Hirata, Y.; Kuriyama, M.; Watanabe, K.; Watanabe, H.; Hongu, N.; Arai, N. Single ingestion of trehalose enhances prolonged exercise performance by effective use of glucose and lipid in healthy men. Nutrients 2021, 13, 1439. [Google Scholar] [CrossRef]
- Hamada, N.; Wadazumi, T.; Hirata, Y.; Watanabe, H.; Hongu, N.; Arai, N. Effects of Trehalose Solutions at Different Concentrations on High-Intensity Intermittent Exercise Performance. Nutrients 2022, 14, 1776. [Google Scholar] [CrossRef]





| Mixture Composition | Grams (g) |
|---|---|
| Trehalose | 7.5 |
| Spermidine | 0.00225 |
| Camellia Sinensis e.s. | 0.075 |
| Catechins | 0.0375 |
| Vitamin C | 0.06 |
| Niacin | 0.00375 |
| Silica | 0.225 |
| Microcrystalline cellulose | 0.075 |
| Orange aroma | 0.3 |
| Athletes (n = 5) | Lower/Upper 95% CI of Mean | |
|---|---|---|
| Age (years) | 21.8 ± 1.3 | 20.2–23.4 |
| Sex (M/F) | 2/3 | / |
| Cholesterol (mg/dL) | 162.4 ± 27.9 | 127.8/197.0 |
| BMI | 20.6 ± 2.5 | 17.5/23.7 |
| Glycemia (mg/dL) | 83.6 ± 6.7 | 75.2/91.9 |
| Training per week (h) | 11.8 ± 3.5 | 8.0/15.5 |
| Sport practice (years) | 12.7 ± 4.6 | 8.2/17 |
| Systolic blood pressure (mmHg) | 112.8 ± 4 | 107/117 |
| Diastolic blood pressure (mmHg) | 72 ± 7 | 65/78 |
| Resting heart rate (bpm) | 54 ± 5 | 48/60 |
| Peak heart rate (bpm) | 168 ± 12 | 163–178 |
| Maximum workload (METs) | 12.5 ± 2 | 10.5–14.3 |
| Before Mean ± SD | After Mean ± SD | p-Value | Lower/Upper 95% CI of Mean before | Lower/Upper 95% CI of Mean after | |
|---|---|---|---|---|---|
| CK (mU/mL) | |||||
| No treatment | 483.1 ± 103.4 | 484.3 ± 86.7 | 0.99 | 354.9/611.7 | 376.5/592.0 |
| Mixture treatment | 463.1 ± 102.2 | 322.4 ± 93.9 | ** p < 0.01 | 336.2/590.0 | 205.8/439.1 |
| LDH (mU/mL) | |||||
| No treatment | 119.7 ± 29.8 | 121.9 ± 29.3 | 0.99 | 82.7/156.8 | 85.5/158.2 |
| Mixture treatment | 120.1 ± 40.0 | 72.3 ± 20.3 | * p < 0.05 | 70.42/169.8 | 47.1/97.5 |
| Myoglobin (ng/mL) | |||||
| No treatment | 111.8 ± 25.4 | 112.9 ± 23.6 | 0.99 | 80.3/143.4 | 83.6/142.1 |
| Mixture treatment | 114.9 ± 27.1 | 99.4 ± 21.6 | * p < 0.05 | 81.9/148.6 | 72.5/126.2 |
| sNOX2-dp (pg/mL) | |||||
| No treatment | 17.9 ± 3.5 | 18.8 ± 3.3 | 0.99 | 13.5/22.3 | 14.6/22.8 |
| Mixture treatment | 17.1 ± 5.9 | 8.6 ± 2.4 | ** p < 0.01 | 9.7/24.4 | 5.6/11.6 |
| H2O2 (μM) | |||||
| No treatment | 18.3 ± 2.8 | 19.3 ± 4.8 | 0.99 | 14.7/21.8 | 13.3/25.4 |
| Mixture treatment | 17.8 ± 2.6 | 10.5 ± 2.3 | * p < 0.05 | 14.5/21.1 | 7.6/13.3 |
| HBA (%) | |||||
| No treatment | 38.3 ± 6.6 | 37.7 ± 6.6 | 0.99 | 30.0/46.5 | 29.4/45.9 |
| Mixture treatment | 42.1 ± 7.2 | 52.2 ± 4.1 | * p < 0.05 | 33.2/51.1 | 47.0/57.1 |
| ATG5 (ng/mL) | |||||
| No treatment | 194.5 ± 30.5 | 107.6 ± 37.4 | 0.99 | 66.5/142.4 | 61.1/154.0 |
| Mixture treatment | 108.0 ± 23.7 | 155.9 ± 37.5 | * p < 0.05 | 78.5/137.5 | 109.4/202.4 |
| P62 (ng/mL) | |||||
| No treatment | 93.7 ± 6.1 | 93.1 ± 9.6 | 0.99 | 86.1/101.3 | 81.2/105.1 |
| Mixture treatment | 90.9 ± 13.9 | 71.8 ± 16.6 | ** p < 0.01 | 73.7/108.3 | 51.3/92.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, A.; Fossati, C.; Pigozzi, F.; Borrione, P.; Peruzzi, M.; Bartimoccia, S.; Saba, F.; Pingitore, A.; Biondi-Zoccai, G.; Petramala, L.; et al. Natural Activators of Autophagy Reduce Oxidative Stress and Muscle Injury Biomarkers in Endurance Athletes: A Pilot Study. Nutrients 2023, 15, 459. https://doi.org/10.3390/nu15020459
D’Amico A, Fossati C, Pigozzi F, Borrione P, Peruzzi M, Bartimoccia S, Saba F, Pingitore A, Biondi-Zoccai G, Petramala L, et al. Natural Activators of Autophagy Reduce Oxidative Stress and Muscle Injury Biomarkers in Endurance Athletes: A Pilot Study. Nutrients. 2023; 15(2):459. https://doi.org/10.3390/nu15020459
Chicago/Turabian StyleD’Amico, Alessandra, Chiara Fossati, Fabio Pigozzi, Paolo Borrione, Mariangela Peruzzi, Simona Bartimoccia, Filippo Saba, Annachiara Pingitore, Giuseppe Biondi-Zoccai, Luigi Petramala, and et al. 2023. "Natural Activators of Autophagy Reduce Oxidative Stress and Muscle Injury Biomarkers in Endurance Athletes: A Pilot Study" Nutrients 15, no. 2: 459. https://doi.org/10.3390/nu15020459
APA StyleD’Amico, A., Fossati, C., Pigozzi, F., Borrione, P., Peruzzi, M., Bartimoccia, S., Saba, F., Pingitore, A., Biondi-Zoccai, G., Petramala, L., De Grandis, F., Vecchio, D., D’Ambrosio, L., Schiavon, S., Sciarra, L., Nocella, C., & Cavarretta, E. (2023). Natural Activators of Autophagy Reduce Oxidative Stress and Muscle Injury Biomarkers in Endurance Athletes: A Pilot Study. Nutrients, 15(2), 459. https://doi.org/10.3390/nu15020459