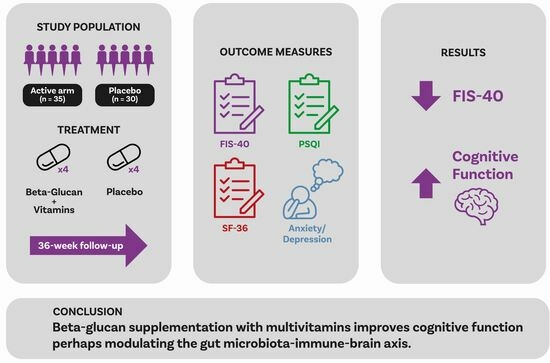
Yeast Beta-Glucan Supplementation with Multivitamins Attenuates Cognitive Impairments in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention Protocol
2.3. Testing of Dietary Supplements
2.4. Study Design and Procedures
2.5. Primary Endpoint
Fatigue Perception
2.6. Secondary Endpoints
2.6.1. Sleep Quality
2.6.2. Anxiety and Depression
2.6.3. Health-Related Quality of Life
2.7. Sample Size Estimation and Power Analysis
2.8. Compliance Monitoring and Adverse Events
2.9. Statistical Analysis
2.9.1. Independent Sample Analysis
2.9.2. Paired Data Analysis
2.9.3. Categorical Data Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Changes in Fatigue Perception
3.3. Changes in Sleep Quality, Anxiety/Depression, and Health-Related Quality of Life
3.3.1. Sleep Quality Assessment
3.3.2. Anxiety and Depression
3.3.3. Health-Related Quality of Life
3.4. Clinical Safety and Intervention Tolerability
4. Discussion
Strengths and Limitations
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estevez-Lopez, F.; Mudie, K.; Wang-Steverding, X.; Bakken, I.J.; Ivanovs, A.; Castro-Marrero, J.; Nacul, L.; Alegre, J.; Zalewski, P.; Słomko, J.; et al. Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology. J. Clin. Med. 2020, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.; Saez-Francas, N.; Castro-Marrero, J.; Aliste, L.; Fernandez de Sevilla, T.; Alegre, J. Gender differences in chronic fatigue syndrome. Reumatol. Clin. 2016, 12, 72–77. [Google Scholar] [CrossRef]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina 2021, 57, 510. [Google Scholar] [CrossRef] [PubMed]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Saez-Francas, N.; Santillo, D.; Alegre, J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: All roads lead to Rome. Br. J. Pharmacol. 2017, 174, 345–369. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Faro, M.; Zaragoza, M.C.; Aliste, L.; de Sevilla, T.F.; Alegre, J. Unemployment and work disability in individuals with chronic fatigue syndrome/myalgic encephalomyelitis: A community-based cross-sectional study from Spain. BMC Public Health 2019, 19, 840. [Google Scholar] [CrossRef]
- Roberts, D. Chronic fatigue syndrome and quality of life. Patient Relat. Outcome Meas. 2018, 9, 253–262. [Google Scholar] [CrossRef]
- Fernandez-Quiros, J.; Lacasa-Cazcarra, M.; Alegre-Martin, J.; Sanmartin-Sentanes, R.; Almirall, M.; Launois-Obregon, P.; Castro-Marrero, J.; Rodríguez-Urrutia, A.; Navarro-Sanchis, J.A.; Ramos-Quiroga, J.A. The Conners Continuous Performance Test CPT3TM: Is it a reliable marker to predict neurocognitive dysfunction in Myalgic encephalomyelitis/chronic fatigue syndrome? Front. Psychol. 2023, 14, 1127193. [Google Scholar] [CrossRef]
- Jain, V.; Arunkumar, A.; Kingdon, C.; Lacerda, E.; Nacul, L. Prevalence of and risk factors for severe cognitive and sleep symptoms in ME/CFS and MS. BMC Neurol. 2017, 17, 117. [Google Scholar] [CrossRef]
- Wirth, K.J.; Scheibenbogen, C.; Paul, F. An attempt to explain the neurological symptoms of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Transl. Med. 2021, 19, 471. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- What’s in a name? Systemic exertion intolerance disease. Lancet 2015, 385, 663. [CrossRef] [PubMed]
- Cvejic, E.; Birch, R.C.; Vollmer-Conna, U. Cognitive Dysfunction in Chronic Fatigue Syndrome: A Review of Recent Evidence. Curr. Rheumatol. Rep. 2016, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Saez-Francas, N.; Segundo, M.J.; Calvo, N.; Faro, M.; Aliste, L.; de Sevilla, T.F.; Alegre, J. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome—A randomized, controlled, double-blind trial. Clin. Nutr. 2016, 35, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Twisk, F.N. Why myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: Disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuroendocrinol. Lett. 2009, 30, 677–693. [Google Scholar]
- Castro-Marrero, J.; Zaragoza, M.C.; Gonzalez-Garcia, S.; Aliste, L.; Saez-Francas, N.; Romero, O.; Ferré, A.; Fernandez de Sevilla, T.; Alegre, J. Poor self-reported sleep quality and health-related quality of life in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J. Sleep Res. 2018, 27, e12703. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Gut inflammation in chronic fatigue syndrome. Nutr. Metab. 2010, 7, 79. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Wallis, A.; Ball, M.; Butt, H.; Lewis, D.P.; McKechnie, S.; Paull, P.; Jaa-Kwee, A.; Bruck, D. Open-label pilot for treatment targeting gut dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: Neuropsychological symptoms and sex comparisons. J. Transl. Med. 2018, 16, 24. [Google Scholar] [CrossRef]
- Martin, F.; Blanco-Suarez, M.; Zambrano, P.; Caceres, O.; Almirall, M.; Alegre-Martin, J.; Lobo, B.; González-Castro, A.M.; Santos, J.; Domingo, J.C.; et al. Increased gut permeability and bacterial translocation are associated with fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: Implications for disease-related biomarker discovery. Front. Immunol. 2023, 14, 1253121. [Google Scholar] [CrossRef]
- Guo, C.; Che, X.; Briese, T.; Ranjan, A.; Allicock, O.; Yates, R.A.; Cheng, A.; March, D.; Hornig, M.; Komaroff, A.L.; et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe 2023, 31, 288–304.e288. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; Plichta, D.; Joshi, A.D.; Bonilla, G.; Peng, V.; Colizzo, F.P.; Luther, J.; Khalili, H.; Garber, J.J.; van der Woude, C.J.; et al. Alterations in Fecal Microbiomes and Serum Metabolomes of Fatigued Patients With Quiescent Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 519–527.e5. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; et al. The role of the gut microbiome in cancer-related fatigue: Pilot study on epigenetic mechanisms. Support. Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sanchez-Alcoholado, L.; Martin-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children With Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. beta-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived beta-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef]
- Varesi, A.; Deumer, U.S.; Ananth, S.; Ricevuti, G. The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications. J. Clin. Med. 2021, 10, 5077. [Google Scholar] [CrossRef]
- Konig, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Lober, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2021, 12, 628741. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Yang, Y.; Gao, Y.; Sun, Q.; Liu, J.; Yang, X.; Wang, J.; Zhang, J. beta-glucan Salecan Improves Exercise Performance and Displays Anti-Fatigue Effects through Regulating Energy Metabolism and Oxidative Stress in Mice. Nutrients 2018, 10, 858. [Google Scholar] [CrossRef]
- Dharsono, T.; Rudnicka, K.; Wilhelm, M.; Schoen, C. Effects of Yeast (1,3)-(1,6)-Beta-Glucan on Severity of Upper Respiratory Tract Infections: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Subjects. J. Am. Coll. Nutr. 2019, 38, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ganda Mall, J.P.; Casado-Bedmar, M.; Winberg, M.E.; Brummer, R.J.; Schoultz, I.; Keita, A.V. A beta-Glucan-Based Dietary Fiber Reduces Mast Cell-Induced Hyperpermeability in Ileum From Patients With Crohn’s Disease and Control Subjects. Inflamm. Bowel Dis. 2017, 24, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Tanaka, K.; Suemasu, S.; Ishihara, T.; Tahara, K.; Suzuki, T.; Suzuki, H.; Fukudo, S.; Mizushima, T. Effects of beta-(1,3-1,6)-D-glucan on irritable bowel syndrome-related colonic hypersensitivity. Biochem. Biophys. Res. Commun. 2012, 420, 444–449. [Google Scholar] [CrossRef]
- Ciacci, C.; Franceschi, F.; Purchiaroni, F.; Capone, P.; Buccelletti, F.; Iacomini, P.; Ranaudo, A.; Andreozzi, P.; Tondi, P.; Gentiloni Siveri, N.; et al. Effect of beta-glucan, inositol and digestive enzymes in GI symptoms of patients with IBS. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 637–643. [Google Scholar]
- Glynn, L.M.; Sandman, C.A. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom. Med. 2014, 76, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Muratsubaki, T.; Van Oudenhove, L.; Morishita, J.; Yoshizawa, M.; Kohno, K.; Yagihashi, M.; Tanaka, Y.; Mugikura, S.; Dupont, P.; et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci. Rep. 2017, 7, 12425. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18 (Suppl. S1), S79–S83. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Alonso, J.; Prieto, L.; Anto, J.M. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results. Med. Clin. 1995, 104, 771–776. [Google Scholar]
- Davis, J.M.; Murphy, E.A.; Brown, A.S.; Carmichael, M.D.; Ghaffar, A.; Mayer, E.P. Effects of oat beta-glucan on innate immunity and infection after exercise stress. Med. Sci. Sport. Exerc. 2004, 36, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The Microbiota-Gut-Brain Axis: Psychoneuroimmunological Insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Gunter, C.; Fleming, E.; Vernon, S.D.; Bateman, L.; Unutmaz, D.; Oh, J. Multi-’omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microbe 2023, 31, 273–287.e275. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suarez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public. Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Germain, A.; Giloteaux, L.; Moore, G.E.; Levine, S.M.; Chia, J.K.; Keller, B.A.; Stevens, J.; Franconi, C.J.; Mao, X.; Shungu, D.C.; et al. Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight 2022, 7, e157621. [Google Scholar] [CrossRef]
- Joseph, P.; Singh, I.; Oliveira, R.; Capone, C.A.; Mullen, M.P.; Cook, D.B.; Stovall, M.C.; Squires, J.; Madsen, K.; Waxman, A.B.; et al. Exercise Pathophysiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Postacute Sequelae of SARS-CoV-2: More in Common Than Not? Chest 2023, 164, 717–726. [Google Scholar] [CrossRef]
- Smith, A.; Bazzoni, C.; Beale, J.; Elliott-Smith, J.; Tiley, M. High fibre breakfast cereals reduce fatigue. Appetite 2001, 37, 249–250. [Google Scholar] [CrossRef]
- Buigues, C.; Fernandez-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martinez, R.; Martinez-Martinez, M.; Verdejo, Y.; Mascarós, M.C.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Rahn, M.; Dioum, E.H.; Jenkins, A.L.; Ezatagha, A.; Campbell, J.E.; Chu, Y. Effect of Oat beta-Glucan on Affective and Physical Feeling States in Healthy Adults: Evidence for Reduced Headache, Fatigue, Anxiety and Limb/Joint Pains. Nutrients 2021, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- Weitberg, A.B. A phase I/II trial of beta-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J. Exp. Clin. Cancer Res. 2008, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.S.; Motivala, S.; Olmstead, R.; Irwin, M.R. Sleep depth and fatigue: Role of cellular inflammatory activation. Brain Behav. Immun. 2011, 25, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. beta-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, P.; Wang, R.; Zhou, M.; Pang, N.; Cui, X.; Ge, X.; Liu, X.; Huang, X.F.; Yu, Y. Three Different Types of beta-Glucans Enhance Cognition: The Role of the Gut-Brain Axis. Front. Nutr. 2022, 9, 848930. [Google Scholar] [CrossRef]



| Variables | Active (n = 29) | Placebo (n = 22) | p-Values |
|---|---|---|---|
| Age (years) | 52.90 ± 6.47 | 52.50 ± 7.48 | 0.90 |
| BMI (kg/m2) | 23.58 ± 3.15 | 22.95 ± 2.83 | 0.39 |
| Heart rate (bpm) | 75.27 ± 11.69 | 74.50 ± 7.71 | 1.00 |
| Concomitant drugs | |||
| Anticonvulsants | 9 | 6 | 0.98 |
| Antidepressants | 24 | 16 | 0.60 |
| Anxiolytics | 19 | 14 | 0.87 |
| Analgesics | 21 | 13 | 0.48 |
| NSAIDs | 6 | 5 | 0.86 |
| FIS-40 Domains | Baseline | 36-Weeks | p-Values 1 |
|---|---|---|---|
| Active arm (n = 29) | |||
| Cognitive | 33.31 ± 7.03 | 31.45 ± 9.76 | 0.0338 * |
| Psychosocial | 61.79 ± 14.59 | 60.45 ± 16.92 | 0.3681 |
| Physical functioning | 34.52 ± 5.20 | 33.28 ± 6.78 | 0.0725 |
| Total FIS-40 score | 129.62 ± 25.37 | 125.17 ± 31.43 | 0.1070 |
| Placebo (n = 22) | |||
| Cognitive | 33.82 ± 5.42 | 32.73 ± 5.76 | 0.3725 |
| Psychosocial | 64.14 ± 11.79 | 59.45 ± 12.92 | 0.1398 |
| Physical functioning | 33.73 ± 5.90 | 32.32 ± 6.95 | 0.1313 |
| Total FIS-40 score | 131.68 ± 21.16 | 124.50 ± 23.83 | 0.1375 |
| PSQI Domains | Baseline | 36 Weeks | p-Values 1 |
|---|---|---|---|
| Active arm (n = 29) | |||
| Subjective sleep quality | 2.14 ± 0.88 | 2.17 ± 0.89 | 0.7389 |
| Sleep latency | 2.07 ± 1.03 | 2.14 ± 1.16 | 0.5271 |
| Sleep duration | 1.69 ± 1.00 | 1.62 ± 0.98 | 0.4795 |
| Habitual sleep efficiency | 1.93 ± 1.33 | 1.83 ± 1.20 | 0.5728 |
| Sleep disturbances | 2.10 ± 0.72 | 2.21 ± 0.73 | 0.1797 |
| Use of sleeping medication | 2.07 ± 1.31 | 2.00 ± 1.39 | 0.6256 |
| Daytime dysfunction * | 2.31 ± 0.89 | 2.28 ± 0.75 | 0.7963 |
| Overall PSQI score | 14.31 ± 4.91 | 14.24 ± 4.93 | 0.8845 |
| Placebo (n = 22) | |||
| Subjective sleep quality | 2.05 ± 0.84 | 1.68 ± 0.99 | 0.0588 |
| Sleep latency | 2.32 ± 0.78 | 2.18 ± 0.85 | 0.3173 |
| Sleep duration | 1.41 ± 1.05 | 1.36 ± 1.09 | 0.7630 |
| Habitual sleep efficiency | 1.77 ± 1.31 | 1.59 ± 1.40 | 0.2575 |
| Sleep disturbances | 2.09 ± 0.53 | 1.95 ± 0.58 | 0.2568 |
| Use of sleeping medication | 1.95 ± 1.36 | 2.14 ± 1.17 | 0.3795 |
| Daytime dysfunction * | 1.59 ± 0.73 | 1.73 ± 0.83 | 0.4386 |
| Overall PSQI score | 13.18 ± 3.85 | 12.64 ± 4.70 | 0.4567 |
| FIS-40 Domains | Baseline | 36-Weeks | p-Values 1 |
|---|---|---|---|
| Active arm (n = 29) | |||
| Anxiety | 12.86 ± 5.05 | 12.76 ± 4.70 | 0.7940 |
| Depression | 11.86 ± 5.11 | 11.86 ± 5.59 | 0.5354 |
| Total HADS | 24.72 ± 9.60 | 24.62 ± 9.67 | 0.6716 |
| Placebo (n = 22) | |||
| Anxiety | 11.82 ± 4.55 | 11.00 ± 4.65 | 0.1821 |
| Depression | 11.59 ± 4.07 | 10.68 ± 3.98 | 0.1218 |
| Total HADS | 23.41 ± 8.19 | 21.68 ± 8.20 | 0.1890 |
| SF-36 Domains | Baseline | 36-Weeks | p-Values 1 |
|---|---|---|---|
| Active arm (n = 29) | |||
| Physical functioning | 38.45 ± 20.49 | 40.17 ± 22.50 | 0.8178 |
| Physical role functioning | 6.03 ± 16.66 | 10.34 ± 20.61 | 0.2020 |
| Bodily pain | 15.93 ± 15.58 | 20.90 ± 18.68 | 0.1468 |
| General health perception | 22.76 ± 14.18 | 21.90 ± 13.19 | 0.5683 |
| Vitality | 12.24 ± 13.53 | 16.55 ± 15.93 | 0.0526 |
| Social role functioning | 32.33 ± 26.63 | 36.64 ± 25.65 | 0.3344 |
| Emotional role functioning | 34.48 ± 46.70 | 28.74 ± 43.39 | 0.1695 |
| Mental health status | 38.21 ± 21.18 | 42.07 ± 22.69 | 0.1393 |
| Placebo (n = 22) | |||
| Physical functioning | 39.32 ± 21.17 | 38.18 ± 20.21 | 0.9243 |
| Physical role functioning | 1.14 ± 5.33 | 4.55 ± 16.61 | 0.3287 |
| Bodily pain | 20.14 ± 19.22 | 23.73 ± 15.33 | 0.1089 |
| General health perception | 25.32 ± 14.49 | 27.36 ± 17.57 | 0.3225 |
| Vitality | 17.27 ± 16.24 | 15.23 ± 12.77 | 0.5847 |
| Social role functioning | 30.68 ± 20.68 | 42.05 ± 22.34 | 0.0131 * |
| Emotional role functioning | 33.33 ± 44.84 | 37.88 ± 45.19 | 0.4797 |
| Mental health status | 38.73 ± 23.11 | 44.73 ± 20.01 | 0.1918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacasa, M.; Alegre-Martin, J.; Sentañes, R.S.; Varela-Sende, L.; Jurek, J.; Castro-Marrero, J. Yeast Beta-Glucan Supplementation with Multivitamins Attenuates Cognitive Impairments in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 4504. https://doi.org/10.3390/nu15214504
Lacasa M, Alegre-Martin J, Sentañes RS, Varela-Sende L, Jurek J, Castro-Marrero J. Yeast Beta-Glucan Supplementation with Multivitamins Attenuates Cognitive Impairments in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2023; 15(21):4504. https://doi.org/10.3390/nu15214504
Chicago/Turabian StyleLacasa, Marcos, Jose Alegre-Martin, Ramon Sanmartin Sentañes, Luisa Varela-Sende, Joanna Jurek, and Jesus Castro-Marrero. 2023. "Yeast Beta-Glucan Supplementation with Multivitamins Attenuates Cognitive Impairments in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 15, no. 21: 4504. https://doi.org/10.3390/nu15214504
APA StyleLacasa, M., Alegre-Martin, J., Sentañes, R. S., Varela-Sende, L., Jurek, J., & Castro-Marrero, J. (2023). Yeast Beta-Glucan Supplementation with Multivitamins Attenuates Cognitive Impairments in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 15(21), 4504. https://doi.org/10.3390/nu15214504