Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study
Abstract
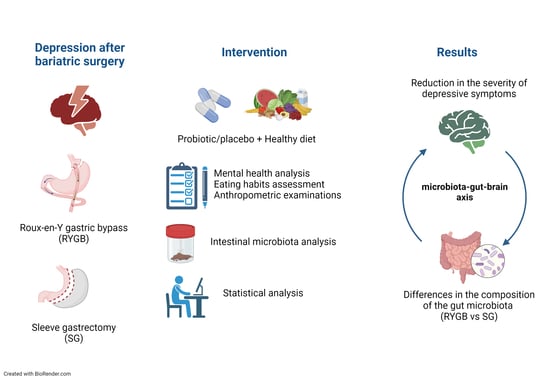
:1. Introduction
2. Materials and Methods
- Probiotic: Sanprobi Barrier (manufacturer: Sanprobi sp. z o. o. sp. k., Szczecin, Poland), consisting of Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Levilactobacillus brevis W63, Lacticaseibacillus casei W56, Ligilactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58;
- Placebo: corn starch, maltodextrins, vegetable protein;
- Dosage: 4 capsules (2 × 109 CFU)/day (taken with a meal (2 capsules in the morning and 2 capsules in the evening);
- The product is available commercially on the Polish market and its composition and dosage have been approved by the relevant health authorities.
2.1. Surgical Techniques
2.2. Anthropometric Examinations
2.3. Survey Research—Mental State
2.4. Eating Habits Assessment
- Variety—consumption of each food group (fish, meat, eggs, legumes, vegetables, fruits, cereals) and different sources of protein (fish, meat, eggs, legumes, dairy products) (0–20 points);
- Adequacy—assessment of consumption of individual food components that need to be supplied to ensure a healthy diet and prevent malnutrition (vegetables, fruits, cereals, fiber, protein, iron, calcium, vitamin C) (0–40 points);
- Moderation—assessing the consumption of dietary components that may require a reduction in daily intake due to an increased risk of developing chronic diseases (total fat, cholesterol, saturated fatty acids, sodium, low calorie foods) (0–30 points);
- Overall dietary balance—assessment of the ratio of individual macronutrients and fatty acids (0–10 points) [45].
2.5. Laboratory Tests
2.6. Markers of the Intestinal Barrier Integrity
2.7. Sequencing Analysis of Bacterial 16S RNA Genes
2.8. Determination of Homocysteine and Vitamin D Levels in Blood Serum
2.9. 16S rRNA Sequence Preprocessing
2.10. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Psychiatric Scales
3.3. DQI-I and Its Subscales
3.4. Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Major, P.; Matłok, M.; Pędziwiatr, M.; Migaczewski, M.; Budzyński, P.; Stanek, M.; Kisielewski, M.; Natkaniec, M.; Budzyński, A. Quality of Life After Bariatric Surgery. Obes. Surg. 2015, 25, 1703–1710. [Google Scholar] [CrossRef]
- Jumbe, S.; Bartlett, C.; Jumbe, S.L.; Meyrick, J. The Effectiveness of Bariatric Surgery on Long Term Psychosocial Quality of Life —A Systematic Review. Obes. Res. Clin. Pract. 2016, 10, 225–242. [Google Scholar] [CrossRef]
- Spirou, D.; Raman, J.; Smith, E. Psychological Outcomes Following Surgical and Endoscopic Bariatric Procedures: A Systematic Review. Obes. Rev. 2020, 21, e12998. [Google Scholar] [CrossRef]
- Canetti, L.; Bachar, E.; Bonne, O. Deterioration of Mental Health in Bariatric Surgery after 10 Years despite Successful Weight Loss. Eur. J. Clin. Nutr. 2016, 70, 17–22. [Google Scholar] [CrossRef]
- Castaneda, D.; Popov, V.B.; Wander, P.; Thompson, C.C. Risk of Suicide and Self-Harm Is Increased After Bariatric Surgery-a Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 322–333. [Google Scholar] [CrossRef]
- Lim, R.B.C.; Zhang, M.W.B.; Ho, R.C.M. Prevalence of All-Cause Mortality and Suicide among Bariatric Surgery Cohorts: A Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1519. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, Depression, Diet, and the Gut Microbiota: Human–Bacteria Interactions at the Core of Psychoneuroimmunology and Nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Limbana, T.; Khan, F.; Eskander, N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus 2020, 12, e9966. [Google Scholar] [CrossRef]
- Komorniak, N.; Martynova-Van Kley, A.; Nalian, A.; Wroński, M.; Kaseja, K.; Kowalewski, B.; Kaźmierczak-Siedlecka, K.; Łoniewski, I.; Kaczmarczyk, M.; Podsiadło, K.; et al. Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery-The Cross-Sectional Study. Nutrients 2022, 14, 5372. [Google Scholar] [CrossRef]
- Flux, M.C.; Lowry, C.A. Finding Intestinal Fortitude: Integrating the Microbiome into a Holistic View of Depression Mechanisms, Treatment, and Resilience. Neurobiol. Dis. 2020, 135, 104578. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Misera, A.; Liśkiewicz, P.; Łoniewski, I.; Skonieczna-Żydecka, K.; Samochowiec, J. Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression. Pharmaceuticals 2021, 14, 952. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of Probiotic and Prebiotic vs Placebo on Psychological Outcomes in Patients with Major Depressive Disorder: A Randomized Clinical Trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the Mammalian Brain: When Physiology Meets Pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The Effects of Probiotic Supplementation on Mental Health, Biomarkers of Inflammation and Oxidative Stress in Patients with Psychiatric Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-Derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity 2020, 28, O1–O58. [Google Scholar] [CrossRef] [PubMed]
- Cambi, M.P.C.; Baretta, G.A.P. Bariatric Diet Guide: Plate Model Template for Bariatric Surgery Patients. Arq. Bras. Cir. Dig. 2018, 31, e1375. [Google Scholar] [CrossRef]
- Tyson, C.C.; Nwankwo, C.; Lin, P.-H.; Svetkey, L.P. The Dietary Approaches to Stop Hypertension (DASH) Eating Pattern in Special Populations. Curr. Hypertens. Rep. 2012, 14, 388–396. [Google Scholar] [CrossRef]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Lin, P.-H.; van Vliet, S.; Lin, C.-Y.; Svetkey, L.; Tyson, C.; Scialla, J. Impact of the DASH Diet on Intestinal Permeability and Inflammation Markers. Curr. Dev. Nutr. 2020, 4, 542. [Google Scholar] [CrossRef]
- Steenackers, N.; Van der Schueren, B.; Mertens, A.; Lannoo, M.; Grauwet, T.; Augustijns, P.; Matthys, C. Iron Deficiency after Bariatric Surgery: What Is the Real Problem? Proc. Nutr. Soc. 2018, 77, 445–455. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Komorniak, N.; Szczuko, M.; Kowalewski, B.; Stachowska, E. Nutritional Deficiencies, Bariatric Surgery, and Serum Homocysteine Level: Review of Current Literature. Obes. Surg. 2019, 29, 3735–3742. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a Multi-Strain Probiotic Supplement for 12 Weeks in Circulating Endotoxin Levels and Cardiometabolic Profiles of Medication Naïve T2DM Patients: A Randomized Clinical Trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-Month Multi-Strain Probiotics Supplementation in Endotoxemic, Inflammatory and Cardiometabolic Status of T2DM Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.; Szulińska, M.; Łoniewski, I.; Kręgielska-Narożna, M.; Skonieczna-Żydecka, K.; Kosciolek, T.; Bezshapkin, V.; Bogdański, P. Treatment With Multi-Species Probiotics Changes the Functions, Not the Composition of Gut Microbiota in Postmenopausal Women With Obesity: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Cell Infect. Microbiol. 2022, 12, 815798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Gorenstein, C. Assessment of Depression in Medical Patients: A Systematic Review of the Utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Kapci, E.G.; Uslu, R.; Turkcapar, H.; Karaoglan, A. Beck Depression Inventory II: Evaluation of the Psychometric Properties and Cut-off Points in a Turkish Adult Population. Depress. Anxiety 2008, 25, E104–E110. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Pucci, A.; Batterham, R.L. Mechanisms Underlying the Weight Loss Effects of RYGB and SG: Similar, yet Different. J. Endocrinol. Investig. 2019, 42, 117–128. [Google Scholar] [CrossRef]
- Benaiges, D.; Más-Lorenzo, A.; Goday, A.; Ramon, J.M.; Chillarón, J.J.; Pedro-Botet, J.; Roux, J.A.F.-L. Laparoscopic Sleeve Gastrectomy: More than a Restrictive Bariatric Surgery Procedure? World J. Gastroenterol. 2015, 21, 11804–11814. [Google Scholar] [CrossRef]
- Schauer, P.R.; Ikramuddin, S.; Hamad, G.; Eid, G.M.; Mattar, S.; Cottam, D.; Ramanathan, R.; Gourash, W. Laparoscopic Gastric Bypass Surgery: Current Technique. J. Laparoendosc. Adv. Surg. Tech. 2003, 13, 229–239. [Google Scholar] [CrossRef]
- Sirajudeen, M.S.; Dilshad Manzar, M.; Alqahtani, M.; Alzhrani, M.; Albougami, A.; Somasekharan Pillai, P.; Spence, D.W.; Pandi-Perumal, S.R. Psychometric Properties of the Athens Insomnia Scale in Occupational Computer Users. Healthcare 2020, 8, 89. [Google Scholar] [CrossRef]
- Hamilton, M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity Classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) Provides an Effective Tool for Cross-National Comparison of Diet Quality as Illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.r-project.org/ (accessed on 24 August 2023).
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the Analysis of High-Throughput Sequencing Datasets: Characterizing RNA-Seq, 16S rRNA Gene Sequencing and Selective Growth Experiments by Compositional Data Analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Alsubaie, S.; Asiri, G.; Asiri, E.; Alqahtani, F.; Bredy, G.; Alshehri, D. Depression and Anxiety on Post-Bariatric Surgery among Saudi Adults Residing in Abha, Asir Province, Saudi Arabia. Int. J. Med. Dev. Ctries. 2021, 5, 165–171. [Google Scholar] [CrossRef]
- Brown, R.M.; Guerrero-Hreins, E.; Brown, W.A.; le Roux, C.W.; Sumithran, P. Potential Gut–Brain Mechanisms behind Adverse Mental Health Outcomes of Bariatric Surgery. Nat. Rev. Endocrinol. 2021, 17, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Gentile, J.K.A.; Oliveira, K.D.; Pereira, J.G.; Tanaka, D.Y.; Guidini, G.N.; Cadona, M.Z.; Siriani-Ribeiro, D.W.; Perondini, M.T. The Intestinal Microbiome in Patients Undergoing Bariatric Surgery: A Systematic Review. Arq. Bras. Cir. Dig. 2022, 35, e1707. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.-H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C.; et al. Microbiota, Metabolome, and Immune Alterations in Obese Mice Fed a High-Fat Diet Containing Type 2 Resistant Starch. Mol. Nutr. Food Res. 2017, 61, 1700184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Coales, I.; Penney, N.; McDonald, J.A.K.; Phetcharaburanin, J.; Seyfried, F.; Li, J.V. A Subset of Roux-En-Y Gastric Bypass Bacterial Consortium Colonizes the Gut of Nonsurgical Rats without Inducing Host-Microbe Metabolic Changes. mSystems 2020, 5, e01047-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Teng, T.; Li, X.; Fan, L.; Xiang, Y.; Jiang, Y.; Du, K.; Zhang, Y.; Zhou, X.; Xie, P. Impact of Inosine on Chronic Unpredictable Mild Stress-Induced Depressive and Anxiety-Like Behaviors With the Alteration of Gut Microbiota. Front. Cell Infect. Microbiol. 2021, 11, 697640. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Shao, D.; Ma, S.; Wei, W. Altered Fecal Microbiota Signatures in Patients With Anxiety and Depression in the Gastrointestinal Cancer Screening: A Case-Control Study. Front. Psychiatry 2021, 12, 757139. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Bao, Z.; Gui, X.; Li, A.N.; Yang, Z.; Li, M.D. Clostridiales Are Predominant Microbes That Mediate Psychiatric Disorders. J. Psychiatr. Res. 2020, 130, 48–56. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of Human Colonic Butyrate-Producing Bacteria Revealed by Analysis of the Butyryl-CoA:Acetate CoA-Transferase Gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K. Gut Microbiota and Hepatic Encephalopathy. Metab. Brain Dis. 2013, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.G.; Kluge, H.; Hirche, F.; Kiowski, A.; Schutkowski, A.; Corrent, E.; Bartelt, J.; König, B.; Stangl, G.I. High Leucine Diets Stimulate Cerebral Branched-Chain Amino Acid Degradation and Modify Serotonin and Ketone Body Concentrations in a Pig Model. PLoS ONE 2016, 11, e0150376. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 472. [Google Scholar] [CrossRef]
- Cook, J.; Lehne, C.; Weiland, A.; Archid, R.; Ritze, Y.; Bauer, K.; Zipfel, S.; Penders, J.; Enck, P.; Mack, I. Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation. Nutrients 2020, 12, 2396. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Breymeyer, K.L.; Lampe, J.W.; McGregor, B.A.; Neuhouser, M.L. Subjective Mood and Energy Levels of Healthy Weight and Overweight/Obese Healthy Adults on High-and Low-Glycemic Load Experimental Diets. Appetite 2016, 107, 253–259. [Google Scholar] [CrossRef]
- Muñoz, M.-A.; Fíto, M.; Marrugat, J.; Covas, M.-I.; Schröder, H.; REGICOR and HERMES investigators. Adherence to the Mediterranean Diet Is Associated with Better Mental and Physical Health. Br. J. Nutr. 2009, 101, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and Traditional Diets with Depression and Anxiety in Women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A Modified Mediterranean Dietary Intervention for Adults with Major Depression: Dietary Protocol and Feasibility Data from the SMILES Trial. Nutr. Neurosci. 2018, 21, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Seganfredo, F.B.; Blume, C.A.; Moehlecke, M.; Giongo, A.; Casagrande, D.S.; Spolidoro, J.V.N.; Padoin, A.V.; Schaan, B.D.; Mottin, C.C. Weight-Loss Interventions and Gut Microbiota Changes in Overweight and Obese Patients: A Systematic Review. Obes. Rev. 2017, 18, 832–851. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; del Mar Roca-Rodriguez, M.; Camargo, A.; Ocaña-Wilhelmi, L.; Cardona, F.; Tinahones, F.J. Lipopolysaccharide and Lipopolysaccharide-Binding Protein Levels and Their Relationship to Early Metabolic Improvement after Bariatric Surgery. Surg. Obes. Relat. Dis. 2015, 11, 933–939. [Google Scholar] [CrossRef]
- Yang, P.-J.; Lee, W.-J.; Tseng, P.-H.; Lee, P.-H.; Lin, M.-T.; Yang, W.-S. Bariatric Surgery Decreased the Serum Level of an Endotoxin-Associated Marker: Lipopolysaccharide-Binding Protein. Surg. Obes. Relat. Dis. 2014, 10, 1182–1187. [Google Scholar] [CrossRef]
- Tuomi, K.; Logomarsino, J.V. Bacterial Lipopolysaccharide, Lipopolysaccharide-Binding Protein, and Other Inflammatory Markers in Obesity and After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016, 14, 279–288. [Google Scholar] [CrossRef]





| Placebo | Probiotic | P | Q | |||
|---|---|---|---|---|---|---|
| n | Mean ± sd, n (%) | n | Mean ± sd, n (%) | |||
| age | 17 | 44.4 ± 10.4 | 21 | 44.9 ± 10.7 | 0.659 | 0.843 |
| Beck scale | 17 | 18.2 ± 7.1 | 21 | 18.8 ± 8.4 | 0.952 | 0.971 |
| Hamilton scale | 16 | 12.9 ± 4.6 | 17 | 13.3 ± 4.7 | 0.971 | 0.971 |
| Insomnia scale | 16 | 10.5 ± 4.8 | 17 | 10.1 ± 3.1 | 0.612 | 0.843 |
| Waist circumference (cm) | 17 | 100.6 ± 11.4 | 21 | 96.2 ± 12.7 | 0.277 | 0.665 |
| WHR | 17 | 0.86 ± 0.07 | 21 | 0.85 ± 0.09 | 0.702 | 0.843 |
| Weight (kg) | 17 | 93.2 ± 18.8 | 21 | 84.8 ± 15.5 | 0.168 | 0.589 |
| FFM (kg) | 17 | 59.3 ± 10.0 | 21 | 57.1 ± 10.4 | 0.463 | 0.788 |
| Fat mass (kg) | 17 | 33.9 + 11.5 | 21 | 29.1 ± 8.2 | 0.191 | 0.589 |
| BMI (kg/m2) | 17 | 32.2 ± 5.3 | 21 | 30.1 ± 4.5 | 0.127 | 0.589 |
| Weight at surgery day (kg) | 17 | 125.9 ± 24.2 | 21 | 114.5 ± 16.0 | 0.167 | 0.589 |
| Time after surgery (months) | 17 | 41.3 ± 41.8 | 21 | 28.4 ± 27.4 | 0.462 | 0.788 |
| LBP (ng/mL) | 17 | 551 ± 127 | 21 | 643 ± 236 | 0.127 | 0.589 |
| LPS (pg/mL) | 17 | 107.9 ± 33.4 | 21 | 97.9 ± 36.6 | 0.252 | 0.665 |
| Homocysteine (nmol/mL) | 17 | 8.4 ± 10.4 | 21 | 8.6 ± 8.8 | 0.411 | 0.788 |
| Zonulin (ng/mL) | 14 | 145.7 ± 84.6 | 15 | 128.9 ± 63.4 | 0.777 | 0.847 |
| Occludin (ng/mL) | 17 | 13.2 ± 3.4 | 21 | 14.1 ± 3.6 | 0.500 | 0.788 |
| Vitamin D (ng/mL) | 17 | 19.4 ±7.9 | 19 | 20.3 ± 6.5 | 0.318 | 0.694 |
| DQI_I (points) | 17 | 46.1 ± 9.7 | 21 | 48.6 ± 7.5 | 0.186 | 0.589 |
| Variety_DQI_I (points) | 17 | 9.6 ± 4.8 | 21 | 10.0 ± 4.9 | 0.677 | 0.843 |
| Adequacy_DQI_I (points) | 17 | 22.5 ± 3.6 | 21 | 22.7 ± 3.5 | 0.746 | 0.847 |
| Moderation_DQI_I (points) | 17 | 13.2 ± 4.8 | 21 | 15.3 ± 4.3 | 0.196 | 0.589 |
| Overall_balance_DQI_I (points) | 17 | 0.71 ± 1.99 | 21 | 0.57 ± 0.93 | 0.196 | 0.788 |
| Surgery type (RYGB/SG) | 17 | 4 (23.5)/13 (76.5) | 21 | 12 (57.1)/9 (42.9) | 0.052 | 0.589 |
| Outcome | Surgery | Intervention | Est | SE | p | Estpairwise | SEpairwise | Ppairwise |
|---|---|---|---|---|---|---|---|---|
| BDI | RYGB | Placebo | −8.00 | 3.49 | 0.022 | −0.95 | 4.11 | 0.817 |
| Probiotic | −7.05 | 2.17 | 0.001 | |||||
| SG | Placebo | −8.55 | 2.69 | 0.002 | 3.22 | 4.25 | 0.449 | |
| Probiotic | −11.76 | 3.29 | <0.001 | |||||
| HRSD | RYGB | Placebo | −6.43 | 3.86 | 0.096 | −2.40 | 4.59 | 0.601 |
| Probiotic | −4.03 | 2.49 | 0.106 | |||||
| SG | Placebo | −2.15 | 2.71 | 0.428 | 5.66 | 4.42 | 0.201 | |
| Probiotic | −7.80 | 3.50 | 0.026 | |||||
| Insomnia | RYGB | Placebo | −3.76 | 2.92 | 0.199 | −1.54 | 3.48 | 0.658 |
| Probiotic | −2.21 | 1.89 | 0.242 | |||||
| SG | Placebo | −3.54 | 2.09 | 0.090 | −3.05 | 3.40 | 0.370 | |
| Probiotic | −0.49 | 2.68 | 0.855 |
| Outcome | Surgery | Intervention | Est | SE | p | Estpairwise | SEpairwise | Ppairwise |
|---|---|---|---|---|---|---|---|---|
| DQI-I | RYGB | Placebo | 23.5 | 4.12 | <0.001 | 6.64 | 4.85 | 0.171 |
| Probiotic | 16.9 | 2.55 | <0.001 | |||||
| SG | Placebo | 10.1 | 3.14 | 0.001 | −6.02 | 4.95 | 0.224 | |
| Probiotic | 16.1 | 3.83 | <0.001 | |||||
| DQI-I variety | RYGB | Placebo | 0.25 | 2.38 | 0.916 | −1.54 | 2.80 | 0.582 |
| Probiotic | 1.79 | 1.47 | 0.224 | |||||
| SG | Placebo | −2.36 | 1.81 | 0.192 | −0.37 | 2.86 | 0.897 | |
| Probiotic | −1.99 | 2.21 | 0.268 | |||||
| DQI-I adequacy | RYGB | Placebo | 10.75 | 1.64 | <0.001 | 3.37 | 1.94 | 0.082 |
| Probiotic | 7.39 | 1.02 | <0.001 | |||||
| SG | Placebo | 4.98 | 1.26 | <0.001 | −2.06 | 1.99 | 0.300 | |
| Probiotic | 7.04 | 1.54 | <0.001 | |||||
| DQI-I moderation | RYGB | Placebo | 7.50 | 2.89 | 0.009 | 2.05 | 3.38 | 0.544 |
| Probiotic | 5.45 | 1.75 | 0.002 | |||||
| SG | Placebo | 5.38 | 2.02 | 0.008 | −0.28 | 3.18 | 0.929 | |
| Probiotic | 5.67 | 2.46 | 0.021 | |||||
| DQI-I overall balance | RYGB | Placebo | 5.00 | 1.09 | <0.001 | 2.08 | 1.27 | 0.103 |
| Probiotic | 2.92 | 0.66 | <0.001 | |||||
| SG | Placebo | 3.69 | 0.77 | <0.001 | 0.61 | 1.21 | 0.614 | |
| Probiotic | 3.08 | 0.94 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorniak, N.; Kaczmarczyk, M.; Łoniewski, I.; Martynova-Van Kley, A.; Nalian, A.; Wroński, M.; Kaseja, K.; Kowalewski, B.; Folwarski, M.; Stachowska, E. Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study. Nutrients 2023, 15, 4905. https://doi.org/10.3390/nu15234905
Komorniak N, Kaczmarczyk M, Łoniewski I, Martynova-Van Kley A, Nalian A, Wroński M, Kaseja K, Kowalewski B, Folwarski M, Stachowska E. Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study. Nutrients. 2023; 15(23):4905. https://doi.org/10.3390/nu15234905
Chicago/Turabian StyleKomorniak, Natalia, Mariusz Kaczmarczyk, Igor Łoniewski, Alexandra Martynova-Van Kley, Armen Nalian, Michał Wroński, Krzysztof Kaseja, Bartosz Kowalewski, Marcin Folwarski, and Ewa Stachowska. 2023. "Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study" Nutrients 15, no. 23: 4905. https://doi.org/10.3390/nu15234905
APA StyleKomorniak, N., Kaczmarczyk, M., Łoniewski, I., Martynova-Van Kley, A., Nalian, A., Wroński, M., Kaseja, K., Kowalewski, B., Folwarski, M., & Stachowska, E. (2023). Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study. Nutrients, 15(23), 4905. https://doi.org/10.3390/nu15234905