Effects of Long-Term Administration of Bovine Bone Gelatin Peptides on Myocardial Hypertrophy in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bovine Bone Gelatin Peptides
2.3. Animal Experiment and Sample Collection
2.4. Histological Measurement
2.5. Label-Free Proteomics Analysis
2.6. Western Blotting
2.7. Enzyme-Linked Immuno Sorbent Assay (ELISA)
2.8. Statistical Analysis
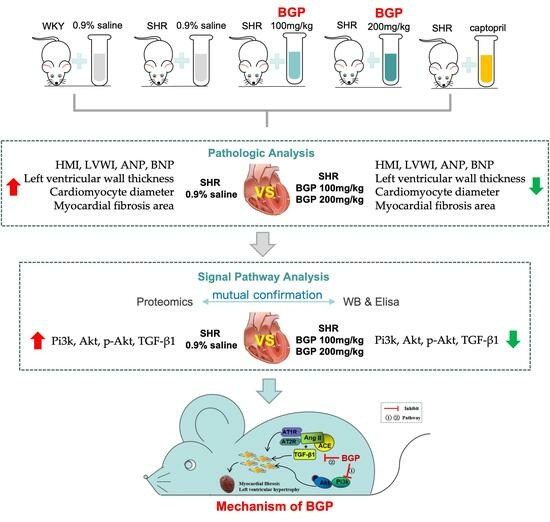
3. Results
3.1. Effects of BGP on Myocardial Hypertrophy and Myocardial Fibrosis in SHR
3.2. Comparison of Proteomics Profiles by Label-Free MS/MS
3.3. GO Annotations Analysis
3.4. Pathway Analysis
3.5. Western Blotting Validation
3.6. Levels of Pi3k/Akt Signaling Pathway and TGF-β1 in SHR Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shenasa, M.; Shenasa, H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int. J. Cardiol. 2017, 237, 60–63. [Google Scholar] [CrossRef]
- You, J.; Wu, J.; Zhang, Q.; Ye, Y.; Wang, S.; Huang, J.; Liu, H.; Wang, X.; Zhang, W.; Bu, L.; et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am. J. Physiol. Circ. Physiol. 2017, 314, H552–H562. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, D.; Sang, M.; Xu, Y.; Liu, Z.; Wu, Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-κB and JAK/STAT signaling pathways in rats. Int. Immunopharmacol. 2017, 48, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-X.; Zhang, P.; Zhang, X.-J.; Zhao, Y.-C.; Deng, K.-Q.; Jiang, X.; Wang, P.-X.; Huang, Z.; Li, H. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat. Commun. 2016, 7, 11267. [Google Scholar] [CrossRef]
- Zou, Y.; Akazawa, H.; Qin, Y.; Sano, M.; Takano, H.; Minamino, T.; Makita, N.; Iwanaga, K.; Zhu, W.; Kudoh, S.; et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 2004, 6, 499–506. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Ferrario, C.M. Cardiac remodelling and RAS inhibition. Ther. Adv. Cardio. Dis. 2016, 10, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Devereux, R.B. Therapeutic options in minimizing left ventricular hypertrophy. Am. Heart J. 2000, 139, s9–s14. [Google Scholar] [CrossRef]
- Julian, D.G.; Pocock, S.J. Effects of long-term use of cardiovascular drugs. Lancet 2015, 385, 325. [Google Scholar] [CrossRef]
- Xing, L.J.; Li, G.H.; Toldra, F.; Zhang, W.G. The physiological activity of bioactive peptides obtained from meat and meat by-products. Adv. Food Nutr. Res. 2021, 97, 97–125. [Google Scholar]
- Cao, S.; Wang, Y.; Hao, Y.; Zhang, W.; Zhou, G. Antihypertensive effects in vitro and in vivo of novel angiotensin-converting enzyme inhibitory peptides from bovine bone gelatin hydrolysate. J. Agr. Food Chem. 2020, 68, 759–768. [Google Scholar] [CrossRef]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. 2019, 59, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Xing, L.J.; Zhang, W.G.; Zhou, G.H. Structure and physical properties of gelatin from bovine bone collagen influenced by acid pretreatment and pepsin. Food Bioprod. Process. 2020, 121, 213–223. [Google Scholar] [CrossRef]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wu, W.; Tian, X.; Hou, M.; Dai, R.; Li, X. Unraveling proteo123me changes of Holstein beef M. semitendinosus and its relationship to meat discoloration during post-mortem storage analyzed by label-free mass spectrometry. J. Proteomics 2017, 154, 85–93. [Google Scholar] [CrossRef]
- Cao, S.M.; Wang, Z.X.; Xing, L.J.; Zhou, L.; Zhang, W.G. Bovine bone gelatin-derived peptides: Food processing characteristics and evaluation of anti-hypertensive and antihyperlipidemic activities. J. Agric. Food Chem. 2022, 70, 9877–9887. [Google Scholar] [CrossRef]
- Edhager, A.V.; Povlsen, J.A.; Løfgren, B.; Bøtker, H.E.; Palmfeldt, J. Proteomics of the rat myocardium during development of Type 2 diabetes mellitus reveals progressive alterations in major metabolic pathways. J. Proteome Res. 2018, 17, 2521–2532. [Google Scholar] [CrossRef]
- Azibani, F.; Fazal, L.; Chatziantoniou, C.; Samuel, J.; Delcayre, C. Aldosterone mediates cardiac fibrosis in the setting of hypertension. Curr. Hypertens. Rep. 2013, 15, 395–400. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Leask, A. Getting to the heart of the matter. Circ. Res. 2015, 116, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.; Wang, H. Fusaric acid (FA) protects heart failure induced by isoproterenol (ISP) in mice through fibrosis prevention via TGF-β1/SMADs and PI3K/AKT signaling pathways. Biomed. Pharmacother. 2017, 93, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Masuyama, T.; Sakata, Y.; Nishikawa, N.; Mano, T.; Yoshida, J.; Miwa, T.; Sugawara, M.; Yamaguchi, Y.; Ookawara, T.; et al. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc. Res. 2002, 55, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Geske, J.B.; McKie, P.M.; Ommen, S.R.; Sorajja, P. B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2013, 61, 2456–2460. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Zhang, Y.; Yang, Y.; Lu, M.; Zhang, J.; Li, S.; Zhang, S.; Li, G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-κB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013, 150, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Olson, E.N. Cardiac plasticity. New Engl. J. Med. 2008, 358, 1370–1380. [Google Scholar] [CrossRef]
- Rapacciuolo, A.; Esposito, G.; Caron, K.; Mao, L.; Thomas, S.A.; Rockman, H.A. Important role of endogenous norepinephrine and epinephrine in the development of in vivo pressure-overload cardiac hypertrophy. J. Am. Coll. Cardiol. 2001, 38, 876–882. [Google Scholar] [CrossRef]
- Gelinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef]
- Naga, P.S.; Esposito, G.; Mao, L.; Koch, W.J.; Rockman, H.A. Gbetagamma-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J. Biol. Chem. 2000, 275, 4693–4698. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Dorn, G.W. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001, 63, 391–426. [Google Scholar] [CrossRef] [PubMed]
- Rockman, H.A.; Koch, W.J.; Lefkowitz, R.J. Seven-transmembrane-spanning receptors and heart function. Nature 2002, 415, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.R. Meeting Koch’s postulates for calcium signaling in cardiac hypertrophy. J. Clin. Investig. 2000, 105, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.R. Stress pathways and heart failure. Cell 1999, 98, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the heart. Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef]
- Kana, K.; Song, H.; Laschinger, C.; Zandstra, P.W.; Radisic, M. PI3K phosphorylation is linked to improved electrical excitability in an in vitro engineered heart tissue disease model system. Tissue Eng. Part A 2015, 21, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Shiojima, I. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Investig. 2005, 115, 2108–2118. [Google Scholar] [CrossRef]
- Nagoshi, T.; Matsui, T.; Aoyama, T.; Leri, A.; Anversa, P.; Li, L.; Ogawa, W.; del Monte, F.; Gwathmey, J.K.; Grazette, L.; et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J. Clin. Investig. 2005, 115, 2128–2138. [Google Scholar] [CrossRef]
- Wohlschlaeger, J.; Schmitz, K.J.; Palatty, J.; Takeda, A.; Takeda, N.; Vahlhaus, C.; Levkau, B.; Stypmann, J.; Schmid, C.; Schmid, K.W.; et al. Roles of cyclooxygenase-2 and phosphorylated Akt (Thr308) in cardiac hypertrophy regression mediated by left-ventricular unloading. J. Thorac. Cardiov. Sur. 2007, 133, 37–43. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Xie, Q. Rosuvastatin inhibits TGF-beta1 expression and alleviates myocardial fibrosis in diabetic rats. Die Pharm. 2013, 68, 355–358. [Google Scholar]
- Leask, A. Potential therapeutic targets for cardiac fibrosis TGF beta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010, 106, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.E.J.; Witt, S.A.; Glascock, B.J.; Nieman, M.J.; Reiser, P.J.; Nix, S.L.; Kimball, T.R.; Doetschman, T. TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Investig. 2002, 109, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chung, A.C.; Huang, X.R.; Lan, H.Y. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-β-dependent and -independent smad pathways: The role of Smad3. Hypertension 2009, 54, 877–884. [Google Scholar] [CrossRef]
- Gao, X.; He, X.; Luo, B.; Peng, L.; Lin, J.; Zuo, Z. Angiotensin II increases collagen I expression via transforming growth factor-beta1 and extracellular signal-regulated kinase in cardiac fibroblasts. Eur. J. Pharmacol. 2009, 606, 115–120. [Google Scholar] [CrossRef]
- Dahlöf, B. Left ventricular hypertrophy and angiotensin II antagonists. Am. J. Hypertens. 2001, 14, 174–182. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Wang, X.; Xing, L.; Zhang, W. Effects of Long-Term Administration of Bovine Bone Gelatin Peptides on Myocardial Hypertrophy in Spontaneously Hypertensive Rats. Nutrients 2023, 15, 5021. https://doi.org/10.3390/nu15245021
Cao S, Wang X, Xing L, Zhang W. Effects of Long-Term Administration of Bovine Bone Gelatin Peptides on Myocardial Hypertrophy in Spontaneously Hypertensive Rats. Nutrients. 2023; 15(24):5021. https://doi.org/10.3390/nu15245021
Chicago/Turabian StyleCao, Songmin, Xinyu Wang, Lujuan Xing, and Wangang Zhang. 2023. "Effects of Long-Term Administration of Bovine Bone Gelatin Peptides on Myocardial Hypertrophy in Spontaneously Hypertensive Rats" Nutrients 15, no. 24: 5021. https://doi.org/10.3390/nu15245021
APA StyleCao, S., Wang, X., Xing, L., & Zhang, W. (2023). Effects of Long-Term Administration of Bovine Bone Gelatin Peptides on Myocardial Hypertrophy in Spontaneously Hypertensive Rats. Nutrients, 15(24), 5021. https://doi.org/10.3390/nu15245021