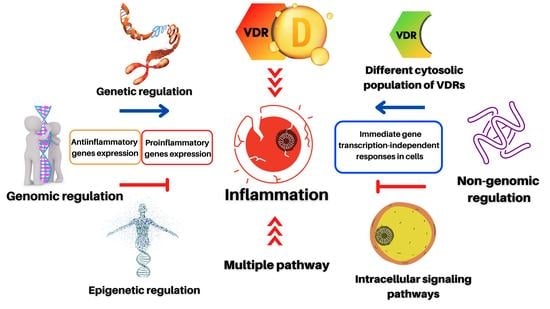
Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases
Abstract
:1. Introduction
2. Pleiotropic Effects of Vitamin D in Inflammatory-Based Diseases
2.1. The Role of Vitamin D in Inflammation at the Cardiovascular Level
2.2. Anti-Inflammatory Role of Vitamin D in the Gastrointestinal System
2.3. Vitamin D Role in Renal Protection
2.4. The Role of Vitamin D in the Nervous System
2.5. The Role of Vitamin D in Autoimmunity
3. Genetic and Epigenetic Regulation of Inflammation by Vitamin D
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pop, T.L.; Sîrbe, C.; Benţa, G.; Mititelu, A.; Grama, A. The role of vitamin D and vitamin D binding protein in chronic liver Diseases. Int. J. Mol. Sci 2022, 23, 10705. [Google Scholar] [CrossRef] [PubMed]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D modulates intestinal microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective properties of vitamin D and lumisterol hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in vitamin D: A statement from the third international conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Vojinovic, J. Vitamin D receptor agonists’ anti-inflammatory properties. Ann. N. Y. Acad. Sci. 2014, 1317, 47–56. [Google Scholar] [CrossRef]
- Sirajudeen, S.; Shah, I.; Al Menhali, A. A narrative role of vitamin D and its receptor: With current evidence on the gastric tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020, 40, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Martín Giménez, V.M.; Inserra, F.; Tajer, C.; Antonietti, L.; Mariani, J.; Manucha, W. Vitamin D supplementation as a rational pharmacological approach in the COVID-19 pandemic. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L941–L948. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Inserra, F.; Ferder, L.; García, J.; Manucha, W. Vitamin D deficiency in African Americans is associated with a high risk of severe disease and mortality by SARS-CoV-2. J. Hum. Hypertens. 2021, 35, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Lahore, H.; Ferder, L.; Holick, M.F.; Manucha, W. The little-explored therapeutic potential of nanoformulations of 1,25-dihydroxyvitamin D3 and its active analogs in prevalent inflammatory and oxidative disorders. Nanomedicine 2021, 16, 2327–2330. [Google Scholar] [CrossRef] [PubMed]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Emerging roles of vitamin D-induced antimicrobial peptides in antiviral innate immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Câmara, A.B.; Brandão, I.A. The relationship between vitamin D deficiency and oxidative stress can be independent of age and gender. Int. J. Vitam. Nutr. Res. 2021, 91, 108–123. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Max, D.; Brandsch, C.; Schumann, S.; Kühne, H.; Frommhagen, M.; Schutkowski, A.; Hirche, F.; Staege, M.S.; Stangl, G.I. Maternal vitamin D deficiency causes smaller muscle fibers and altered transcript levels of genes involved in protein degradation, myogenesis, and cytoskeleton organization in the newborn rat. Mol. Nutr. Food Res. 2014, 58, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Tejada Romero, M.J. Acciones extraóseas de la vitamina D. Rev. Osteoporos. Metab. Miner. 2014, 6, 11–18. [Google Scholar] [CrossRef]
- de Las Heras, N.; Martín Giménez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: Therapeutic effects of vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; Ferder, L.; Inserra, F.; García, J.; Manucha, W. Differences in RAAS/vitamin D linked to genetics and socioeconomic factors could explain the higher mortality rate in African Americans with COVID-19. Ther. Adv. Cardiovasc. Dis. 2020, 14, 1753944720977715. [Google Scholar] [CrossRef]
- Bellia, A.; Garcovich, C.; D’Adamo, M.; Lombardo, M.; Tesauro, M.; Donadel, G.; Gentileschi, P.; Lauro, D.; Federici, M.; Lauro, R.; et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2013, 8, 33–40. [Google Scholar] [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D insufficiency in overweight and obese children and adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; Verrijn Stuart, A.A.; van Summeren, M.; Rakhshandehroo, M.; Nuboer, R.; De Boer, F.K.; Van Den Ham, H.J.; Kalkhoven, E.; Prakken, B.; Schipper, H. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int. J. Obes. 2014, 38, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Chuffa, L.G.A.; Simão, V.A.; Reiter, R.J.; Manucha, W. Protective actions of vitamin D, anandamide and melatonin during vascular inflammation: Epigenetic mechanisms involved. Life Sci. 2022, 288, 120191. [Google Scholar] [CrossRef]
- Teixeira, T.M.; da Costa, D.C.; Resende, A.C.; Soulage, C.O.; Bezerra, F.F.; Daleprane, J.B. Activation of Nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J. Nutr. 2017, 147, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Oma, I.; Andersen, J.K.; Lyberg, T.; Molberg, Ø.; Whist, J.; Fagerland, M.; Almdahl, S.; Hollan, I. Plasma vitamin D levels and inflammation in the aortic wall of patients with coronary artery disease with and without inflammatory rheumatic disease. Scand. J. Rheumatol. 2017, 46, 198–205. [Google Scholar] [CrossRef]
- Tay, H.M.; Yeap, W.H.; Dalan, R.; Wong, S.C.; Hou, H.W. Increased monocyte-platelet aggregates and monocyte-endothelial adhesion in healthy individuals with vitamin D deficiency. FASEB J. 2020, 34, 11133–11142. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek-Czajkowska, E.; Mierzyński, R. Could vitamin D be effective in prevention of preeclampsia? Nutrients 2021, 13, 3854. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and vascular disease. Curr. Vasc. Pharmacol. 2021, 19, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef]
- Lee, A.S.; Jung, Y.J.; Thanh, T.N.; Lee, S.; Kim, W.; Kang, K.P.; Park, S.K. Paricalcitol attenuates lipopolysaccharide-induced myocardial inflammation by regulating the NF-κB signaling pathway. Int. J. Mol. Med. 2016, 37, 1023–1029. [Google Scholar] [CrossRef]
- Martínez-Moreno, J.M.; Herencia, C.; de Oca, A.M.; Díaz-Tocados, J.M.; Vergara, N.; Gómez-Luna, M.J.; López-Argüello, S.D.; Camargo, A.; Peralbo-Santaella, E.; Rodríguez-Ortiz, M.E.; et al. High phosphate induces a pro-inflammatory response by vascular smooth muscle cells and modulation by vitamin D derivatives. Clin. Sci. 2017, 131, 1449–1463. [Google Scholar] [CrossRef]
- Diez, E.R.; Altamirano, L.B.; García, I.M.; Mazzei, L.; Prado, N.J.; Fornes, M.W.; Carrión, F.D.C.; Zumino, A.Z.P.; Ferder, L.; Manucha, W. Heart remodeling and ischemia-reperfusion arrhythmias linked to myocardial vitamin d receptors deficiency in obstructive nephropathy are reversed by paricalcitol. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 211–220. [Google Scholar] [CrossRef]
- Sanz, R.L.; Mazzei, L.; Manucha, W. Implications of the transcription factor WT1 linked to the pathologic cardiac remodeling post-myocardial infarction. Clin. Investig. Arterioscler. 2019, 31, 121–127. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Morrissey, J.; Finch, J.L.; Martin, D.R.; Liapis, H.; Akizawa, T.; Slatopolsky, E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J. Am. Soc. Nephrol. JASN 2007, 18, 1796–1806. [Google Scholar] [CrossRef]
- García, I.M.; Altamirano, L.; Mazzei, L.; Fornés, M.; Cuello-Carrión, F.D.; Ferder, L.; Manucha, W. Vitamin D receptor-modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones 2014, 19, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Sanz, R.; Mazzei, L.; Santino, N.; Ingrasia, M.; Manucha, W. Vitamin D-mitochondria cross-talk could modulate the signaling pathway involved in hypertension development: A translational integrative overview. Clin. Investig. Arterioscler. 2020, 32, 144–155. [Google Scholar] [PubMed]
- Martorell, S.; Hueso, L.; Gonzalez-Navarro, H.; Collado, A.; Sanz, M.J.; Piqueras, L. Vitamin D receptor activation reduces angiotensin-ii-induced dissecting abdominal aortic aneurysm in apolipoprotein E-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.L.; Lavie, C.J. Vitamin D metabolism and the implications for atherosclerosis. Adv. Exp. Med. Biol. 2017, 996, 185–192. [Google Scholar]
- Raed, A.; Bhagatwala, J.; Zhu, H.; Pollock, N.K.; Parikh, S.J.; Huang, Y.; Havens, R.; Kotak, I.; Guo, D.-H.; Dong, Y. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: A placebo controlled randomized trial. PLoS ONE 2017, 12, e0188424. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.G. Vitamin D receptor influences intestinal barriers in health and disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Xiang, W.; Kong, J.; Chen, S.; Cao, L.-P.; Qiao, G.; Zheng, W.; Liu, W.; Li, X.; Gardner, D.G.; Li, Y.C. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E125–E132. [Google Scholar] [CrossRef]
- Chau, Y.Y.; Kumar, J. Vitamin D in chronic kidney disease. Indian J. Pediatr. 2012, 79, 1062–1068. [Google Scholar] [CrossRef]
- Dornas, W.C.; Silva, M.E. Animal models for the study of arterial hypertension. J. Biosci. 2011, 36, 731–737. [Google Scholar] [CrossRef]
- Mazzei, L.; García, M.; Calvo, J.P.; Casarotto, M.; Fornés, M.; Abud, M.A.; Cuello-Carrión, D.; Ferder, L.; Manucha, W. Changes in renal WT-1 expression preceding hypertension development. BMC Nephrol. 2016, 17, 34. [Google Scholar] [CrossRef]
- Mazzei, L.; Docherty, N.G.; Manucha, W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones 2015, 20, 893–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascon-Barré, M.; Huet, P.M. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am. J. Physiol. 1983, 244, E266–E271. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Selhorst, A.; Lampe, S.G.; Liu, Y.; Yang, Y.; Lovett-Racke, A.E. Neuron-specific vitamin d signaling attenuates microglia activation and CNS autoimmunity. Front. Neurol. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.G.; Martín Giménez, V.M.; Holick, M.F.; Barrantes, F.J.; Manucha, W. COVID-19 and neurological sequelae: Vitamin D as a possible neuroprotective and/or neuroreparative agent. Life Sci. 2022, 297, 120464. [Google Scholar] [CrossRef]
- Bayat, M.; Kohlmeier, K.A.; Haghani, M.; Haghighi, A.B.; Khalili, A.; Bayat, G.; Hooshmandi, E.; Shabani, M. Co-treatment of vitamin D supplementation with enriched environment improves synaptic plasticity and spatial learning and memory in aged rats. Psychopharmacology 2021, 238, 2297–2312. [Google Scholar] [CrossRef]
- Gáll, Z.; Székely, O. Role of vitamin D in cognitive dysfunction: New molecular concepts and discrepancies between animal and human findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef]
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef]
- Cui, C.; Xu, P.; Li, G.; Qiao, Y.; Han, W.; Geng, C.; Liao, D.; Yang, M.; Chen, D.; Jiang, P. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system. Redox Biol. 2019, 26, 101295. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, J.; Wang, J.; Cui, C.; Jiang, P. Calcitriol alleviates global cerebral ischemia-induced cognitive impairment by reducing apoptosis regulated by VDR/ERK signaling pathway in rat hippocampus. Brain Res. 2019, 1724, 146430. [Google Scholar] [CrossRef]
- Alessio, N.; Belardo, C.; Trotta, M.C.; Paino, S.; Boccella, S.; Gargano, F.; Pieretti, G.; Ricciardi, F.; Marabese, I.; Luongo, L.; et al. Vitamin D deficiency induces chronic pain and microglial phenotypic changes in mice. Int. J. Mol. Sci. 2021, 22, 3604. [Google Scholar] [CrossRef]
- Lang, F.; Ma, K.; Leibrock, C.B. 1,25(OH)2D3 in brain function and neuropsychiatric disease. Neurosignals 2019, 27, 40–49. [Google Scholar] [PubMed]
- Dërmaku-Sopjani, M.; Kurti, F.; Xuan, N.T.; Sopjani, M. Klotho-dependent role of 1,25(OH)2D3 in the brain. Neurosignals 2021, 29, 14–23. [Google Scholar] [PubMed]
- Zech, L.D.; Scherf-Clavel, M.; Daniels, C.; Schwab, M.; Deckert, J.; Unterecker, S.; Herr, A.S. Patients with higher vitamin D levels show stronger improvement of self-reported depressive symptoms in psychogeriatric day-care setting. J. Neural. Transm. 2021, 128, 1233–1238. [Google Scholar] [CrossRef]
- Seyedi, M.; Gholami, F.; Samadi, M.; Djalali, M.; Effatpanah, M.; Yekaninejad, M.S.; Hashemi, R.; Abdolahi, M.; Chamari, M.; Honarvar, N.M. The effect of vitamin D3 supplementation on serum BDNF, dopamine, and serotonin in children with attention-deficit/hyperactivity disorder. CNS Neurol. Disord. Drug Targets 2019, 18, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Bishop, E.L.; Harrison, S.R.; Swift, A.; Cooper, S.C.; Dimeloe, S.K.; Raza, K.; Hewison, M. Autoimmune disease and interconnections with vitamin D. Endocr. Connect. 2022, 11, e210554. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Leung, P.S.; Adamopoulos, I.E.; Gershwin, M.E. The implication of vitamin D and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 217–226. [Google Scholar]
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological role and therapeutic implications of vitamin D in autoimmunity: Focus on chronic autoimmune diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [PubMed]
- Barnes, C.E.; English, D.M.; Cowley, S.M. Acetylation & Co: An expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem. 2019, 63, 97–107. [Google Scholar] [PubMed] [Green Version]
- Rafehi, H.; Balcerczyk, A.; Lunke, S.; Kaspi, A.; Ziemann, M.; Kn, H.; Okabe, J.; Khurana, I.; Ooi, J.; Khan, A.W.; et al. Vascular histone deacetylation by pharmacological HDAC inhibition. Genome Res. 2014, 24, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, S.; Mohammed, S.A.; Lüscher, T.F.; Costantino, S.; Paneni, F. New mechanisms of vascular dysfunction in cardiometabolic patients: Focus on epigenetics. High Blood Press. Cardiovasc. Prev. 2020, 27, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zarzour, A.; Kim, H.W.; Weintraub, N.L. Epigenetic regulation of vascular diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 984–990. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The extraordinary metabolism of vitamin D. eLife 2022, 11, e77539. [Google Scholar] [CrossRef]
- Al-Garawi, A.; Carey, V.J.; Chhabra, D.; Mirzakhani, H.; Morrow, J.; Lasky-Su, J.; Qiu, W.; Laranjo, N.; Litonjua, A.A.; Weiss, S.T. The role of vitamin D in the transcriptional program of human pregnancy. PLoS ONE 2016, 11, e0163832. [Google Scholar] [CrossRef]
- Mulligan, M.L.; Felton, S.K.; Riek, A.E.; Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 2010, 202, e1–e9. [Google Scholar] [CrossRef]
- Meems, L.M.; Mahmud, H.; Buikema, H.; Tost, J.; Michel, S.; Takens, J.; Verkaik-Schakel, R.N.; Vreeswijk-Baudoin, I.; Mateo-Leach, I.V.; van der Harst, P.; et al. Parental vitamin D deficiency during pregnancy is associated with increased blood pressure in offspring via Panx1 hypermethylation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1571. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, X.; Huang, Y.; Li, G.; Wang, Y.; Li, Y.; Sun, C. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia 2014, 57, 2165–2172. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheum. Dis. Clin. N. Am. 2012, 38, 13–27. [Google Scholar] [CrossRef]
- Izzo, M.; Carrizzo, A.; Izzo, C.; Cappello, E.; Cecere, D.; Ciccarelli, M.; Iannece, P.; Damato, A.; Vecchione, C.; Pompeo, F. Vitamin D: Not just bone metabolism but a key player in cardiovascular diseases. Life 2021, 11, 452. [Google Scholar] [CrossRef]
- Apprato, G.; Fiz, C.; Fusano, I.; Bergandi, L.; Silvagno, F. Natural epigenetic modulators of vitamin D receptor. Appl. Sci. 2020, 10, 4096. [Google Scholar] [CrossRef]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ionova, Z.I.; Sergeeva, E.G.; Berkovich, O.A. Genetic and epigenetic factors regulating the expression and function of the vitamin D receptor in patients with coronary artery disease. Russ. J. Cardiol. 2021, 26, 4251. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Bozic, M.; Álvarez, Á.; de Pablo, C.; Sanchez-Niño, M.D.; Ortiz, A.; Dolcet, X.; Encinas, M.; Fernandez, E.; Valdivielso, J.M. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: Implications for atherosclerosis development. PLoS ONE 2015, 10, e0136863. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A. Vitamin D attenuates pro-inflammatory TNF-α cytokine expression by inhibiting NF-ĸB/p65 signaling in hypertrophied rat hearts. J. Physiol. Biochem. 2015, 71, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Gynther, P.; Toropainen, S.; Matilainen, J.M.; Seuter, S.; Carlberg, C.; Väisänen, S. Mechanism of 1α,25-dihydroxyvitamin D(3)-dependent repression of interleukin-12B. Biochim. Biophys. Acta 2011, 1813, 810–818. [Google Scholar] [CrossRef]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef]
- Devaraj, S.; Yun, J.M.; Duncan-Staley, C.R.; Jialal, I. Low vitamin D levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. Am. J. Clin. Pathol. 2011, 135, 429–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Shi, H.; Su, S.; Harshfield, G.A.; Gutin, B.; Snieder, H.; Dong, Y. A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J. Pediatr. 2013, 162, 1004–1009.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holick, M.F.; Mazzei, L.; García Menéndez, S.; Martín Giménez, V.M.; Al Anouti, F.; Manucha, W. Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients 2023, 15, 767. https://doi.org/10.3390/nu15030767
Holick MF, Mazzei L, García Menéndez S, Martín Giménez VM, Al Anouti F, Manucha W. Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients. 2023; 15(3):767. https://doi.org/10.3390/nu15030767
Chicago/Turabian StyleHolick, Michael F., Luciana Mazzei, Sebastián García Menéndez, Virna Margarita Martín Giménez, Fatme Al Anouti, and Walter Manucha. 2023. "Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases" Nutrients 15, no. 3: 767. https://doi.org/10.3390/nu15030767
APA StyleHolick, M. F., Mazzei, L., García Menéndez, S., Martín Giménez, V. M., Al Anouti, F., & Manucha, W. (2023). Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients, 15(3), 767. https://doi.org/10.3390/nu15030767