Yellow Mealworm (Tenebrio molitor) Powder Promotes a High Bioaccessible Protein Fraction and Low Glycaemic Index in Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Characterisation of White Wheat Flour (WWF) and Yellow Mealworm (Tenebrio molitor) Powder (YMP)
2.2.1. Moisture Content
2.2.2. Ash Content and Speciation of Mineral Elements
2.2.3. Total Protein and Amino Acid Profile
2.2.4. Total Lipid and Fatty Acid Profile
2.2.5. Fibres
2.2.6. Carbohydrates
2.2.7. Energy
2.3. Biscuit Making and Sensorial Study Protocols
2.4. Physical–Chemical Analysis of the Selected Biscuits
2.4.1. Firmness
2.4.2. The Diameter (D) and Thickness (T) Ratio
2.4.3. Proximate Analysis and Profiles of Mineral Elements, Fatty Acids, and Amino Acids
2.4.4. Determination of 5-Hydroxymethylfurfural
2.4.5. In Vitro Digestion Studies
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Compositions of White Wheat Flour and Yellow Mealworm Powder
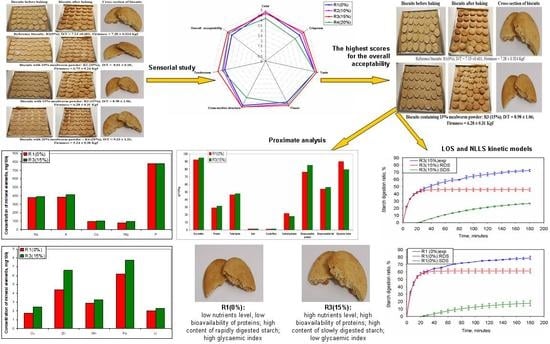
3.2. Sensory Study and Selection of the Biscuits for Further Investigations
3.3. Characterisation of Selected Biscuits
3.3.1. Proximate Composition of Selected Biscuits
3.3.2. In Vitro Digestion of Protein and Fat in Selected Biscuits
3.3.3. Kinetics of Starch Hydrolysis and Estimated Glycaemic Index (eGI) Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goubgou, M.; Songré-Ouattara, L.T.; Bationo, F.; Lingani-Sawadogo, H.; Traoré, Y.; Savadogo, A. Biscuits: A systematic review and meta-analysis of improving the nutritional quality and health benefits. Food Prod. Process. Nutr. 2021, 3, 26–44. [Google Scholar] [CrossRef]
- Wolever, T.M. Carbohydrate and the regulation of blood glucose and metabolism. Nutr. Rev. 2003, 61, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, A.; Rizou, T.; Kiosseoglou, V. Biscuits enriched with dietary fibre powder obtained from the water-extraction residue of maize milling by-product. Plant Foods Hum. Nutr. 2019, 74, 391–398. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Nogueira, A.; Steel, C.J. Protein enrichment of biscuits: A review. Food Rev. Int. 2018, 34, 796–809. [Google Scholar] [CrossRef]
- Lu, L.; He, C.; Liu, B.; Wen, Q.; Xia, S. Incorporation of chickpea flour into biscuits improves the physicochemical properties and in vitro starch digestibility. LWT—Food Sci. Technol. 2022, 159, 113222. [Google Scholar] [CrossRef]
- C/2017/8878. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods; EUR-LEX. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 25 November 2022).
- Global Market Insight. Edible Insects Market; Report ID: GMI501; Global Market Insights Inc.: Selbyville, DE, USA, 2020; Available online: https://www.gminsights.com/industry-analysis/edible-insects-market (accessed on 16 October 2022).
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Zielinska, E.; Pankiewicz, U.; Sujka, M. Nutritional, physiochemical, and biological value of muffins enriched with edible insects flour. Antioxidants 2021, 10, 1122. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Nicula, C.; Peter, A.; Mihaly Cozmuta, L.; Nartea, A.; Kuhalskaya, A.; Pacetti, D.; Silvi, S.; Fiorini, D.; Pruteanu, L. Cricket and yellow mealworm powders promote higher bioaccessible fractions of mineral elements in functional bread. J. Funct. Foods 2022, 99, 105310. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, E.; Pankiewicz, U. Nutritional, physiochemical, and antioxidative characteristics of shortcake biscuits enriched with Tenebrio molitor flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef]
- Bas, A.; Nehir El, S. Nutritional evaluation of biscuits enriched with cricket flour (Acheta domesticus). Int. J. Gastron. Food Sci. 2022, 29, 100583. [Google Scholar] [CrossRef]
- AOAC 925.10-1925; Solids (Total) and Loss on Drying (Moisture) in Flour. AOAC International: Rockville, MD, USA, 1925.
- AOAC 942.05-1995; Determination of Ash in Animal Feed. AOAC International: Rockville, MD, USA, 1925.
- Berghof, MWS-2. Microwave Pressure Digestion Food, Pharma, Cosmetics. 6.0; Application Report Microwave Pressure Digestion, Speed Wave MWS-2; Berghof Products + Instruments GmbH: Eningen, Germany.
- AOAC 945.18-B; Kjeldahl’s Method for Protein Determination in Cereals and Feed. Determination of Total Fat in Flour, Bread, Bakery Product and Pasta with Preliminary Acid Hydrolysis. Determination of Ash in Animal Feed. UDK 127 Operating Manual. AOAC International: Rockville, MD, USA, 2012.
- Vasilica, B.T.B.; Chis, M.S.; Alexa, E.; Pop, C.; Paucean, A.; Man, S.; Igual, M.; Kovacs, M.H.; Kovacs, E.D.; Stanila, S.; et al. The impact of insect flour on sourdough fermentation-fatty acids, amino-acids, minerals and volatile profile. Insects 2022, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- AOAC 920.39.B; Determination of Total Fat in Flour, Bread, Bakery Products and Pasta with Preliminary Acid Hydrolysis, Velp Scientifica—SR 148 Manual, 45. AOAC International: Rockville, MD, USA, 2009.
- Dehelean, A.; Kovacs, E.D.; Magdas, D.A.; Cristea, G.; Voica, C.; Feher, I.; Kovacs, M.H.; Marincas, O.; Zhang, Z.; Li, P.; et al. Infant supplement quality evaluation by inductively coupled plasma-mass spectrometry, gas chromatography, and isotope ratio mass spectrometry. Anal. Lett. 2019, 52, 127–138. [Google Scholar] [CrossRef]
- Commission Regulation (EC) 152/2009 Laying down the Methods of Sampling and Analysis for the Official Control of Feed; EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0152 (accessed on 15 November 2022).
- Montowska, M.; Kowalczewski, P.L.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 29, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumer. Official Journal of the European Union, L 304/18, 22.11.2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 2 December 2022).
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Besir, A.; Yazici, F.; Mortas, M.; Gul, O. A novel spectrophotometric method based on Seliwanoff test to determine 5-(Hydroxymethyl) furfural (HMF) in honey: Development, in house validation and application. LWT—Food Sci. Technol. 2021, 139, 110602. [Google Scholar] [CrossRef]
- Brodkorb, A.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; Clemente, A.; Corredig, M.; Dupont, D.; Dufour, C.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Yusufoğlu, B.; Yama, M.; Karakuş, E. In vitro enzymatic carbohydrate digestion and spectrophotometric glycemic indexes and glycemic loads determination of some Turkish breads. Prog. Nutr. 2021, 23, e2021039. [Google Scholar] [CrossRef]
- Chung, H.J.; Shin, D.H.; Lim, S.T. In vitro starch digestibility and estimated glycemic index of chemically modified corn starches. Food Res. Int. 2008, 41, 579–585. [Google Scholar] [CrossRef]
- Goni, I.; Garcia-Alonso, A.; Calixto, F. A starch hydrolysis procedure to estimate glycemic ındex. Nutr. Res. 1977, 17, 427–437. [Google Scholar] [CrossRef]
- AACC 76–13 (1977) Method. Assay for Analysis of Total Starch in Cereal Products, Total Starch (α-Amylase/Amyloglucosidse) Assay Protocol. Neogen Corporation, Megazim Ltd., 2022. Available online: https://www.megazyme.com/documents/Assay_Protocol/K-TSTA-100A_DATA.pdf (accessed on 15 July 2022).
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–340. [Google Scholar] [CrossRef]
- Huang, R.; Huang, K.; Guan, X.; Zhang, J.; Zhang, P. Incorporation of defatted quinoa flour affects in vitro starch digestion, cooking and rheological properties of wheat noodles. J. Cereal Sci. 2022, 108, 103542. [Google Scholar] [CrossRef]
- Li, C.; Gong, B.; Hu, Y.; Liu, X.; Guan, X.; Zhang, B. Combined crystalline, lamellar and granular structural insights into in vitro digestion rate of native starches. Food Hydrocoll. 2020, 105, 105823. [Google Scholar] [CrossRef]
- Tosi, P.; He, J.; Lovegrove, A.; Gonzáles-Thuillier, I.; Penson, S.; Shewry, P.R. Gradients in compositions in the starchy endosperm of wheat have implications for milling and processing. Trends Food Sci. Technol. 2018, 82, 1–7. [Google Scholar] [CrossRef]
- Nisar, N.; Mustafa, F.; Tahir, A.; Qadri, R.; Yang, Y.; Khan, M.I.; Wang, F. Proximate composition, functional properties and quantitative analysis of benzoyl peroxide and benzoic acid in wheat flour samples: Effect on wheat flour quality. PeerJ 2020, 8, e8788. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Oghbaei, M.; Prakash, J. Effect of fractional milling of wheat on nutritional quality of milled fractions. Trends Carbohydr. Res. 2013, 5, 53–58. [Google Scholar]
- Heshe, G.G.; Haki, G.D.; Woldegiorgis, A.Z.; Gemede, H.F. Effect of conventional milling on the nutritional value and antioxidant capacity of wheat types common in Ethiopia and a recovery attempt with bran supplementation in bread. Food Sci. Nutr. 2016, 4, 534–543. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; Van Loon, J.J. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef]
- Dashefsky, H.S.; Anderson, D.L.; Tobin, E.N.; Peters, T.M. Face fly pupae: A potential feed supplement for poultry. Environ. Entomol. 1976, 5, 680–682. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours. Influence of chemical composition on nutritional and technological aspects. LWT—Food Sci. Technol. 2022, 159, 113220. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Duda-Chodak, A.; Zięć, G. 5-Hydroxymethyl-2-Furfural (HMF)—Heat-induced formation, cccurrence in food and biotransformation—A Review. Pol. J. Food Nutr. Sci. 2013, 63, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Gökmen, V.; Açar, Ö.Ç.; Arda, S.; Francisco, J.M. Effect of leavening agents and sugars on the formation of hydroxymethylfurfural in cookies during baking. Eur. Food Res. Technol. 2008, 226, 1031–1037. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Dietary Reference Values for Phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar] [CrossRef]
- Akimov, M.; Bezuglov, V. Methods of protein digestive stability assay-state of the art. In New Advances in the Basic and Clinical Gastroenterology; Brzozowski, T., Ed.; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Accardo, F.; Miguens-Gomez, A.; Lolli, V.; Faccini, A.; Ardevol, A.; Terra, X.; Caligiani, A.; Pinent, M.; Sforza, S. Molecular composition of lipid and protein fraction of almond, beef and lesser mealworm after in vitro simulated gastrointestinal digestion and correlation with the hormone-stimulating properties of the digesta. Food Res. Int. 2022, 158, 111499. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Mattioli, S.; Paolucci, S.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Paci, G. Effect of cooking techniques on the in vitro protein digestibility, fatty acid profile, and oxidative status of mealworms (Tenebrio molitor). Front. Vet. Sci. 2021, 8, 675572. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Poelaert, C.; Ernens, M.; Liotta, M.; Blecker, C.; Danthine, S.; Tyteca, E.; Haubruge, E.; Alabi, T.; Bindelle, J.; et al. Effect of household cooking techniques on the microbiological load and the nutritional quality of mealworms (Tenebrio molitor L. 1758). Food Res. Int. 2018, 106, 503–508. [Google Scholar] [CrossRef]
- Cao, H.; Wang, C.; Li, R.; Guan, X.; Huang, K.; Zhang, Y. Influence of sprouted oat flour substitution on the texture and in vitro starch digestibility of wheat bread. Food Chem. 2022, 15, 100428. [Google Scholar] [CrossRef]
- Fan, J.-X.; Guo, X.-N.; Zhu, K.-X. Impact of laccase-induced protein cross-linking on the in vitro starch digestion of black highland barley noodles. Food Hydrocoll. 2022, 124, 107298. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trend Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Thorne, M.J.; Wolever, T.M.S.; Jenkins, A.L.; Rao, A.V.; Thompson, L.U. The effect of starch-protein interaction in wheat on the glycemic response and rate of in vitro digestion. Am. J. Clin. Nutr. 1987, 45, 946–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; He, X.-W.; Zhang, B.; Fu, X.; Jane, J.-l.; Huang, Q. Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. Int. J. Biol. Macromol. 2017, 104, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Chen, W.L. Plant fiber. Carbohydrate and lipid metabolism. Am. J. Clin. Nutr. 1979, 32, 346–363. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | Dough and Corresponding Biscuits’ Formulation and Coding (Ratio of Yellow Mealworm Powder *) | |||
|---|---|---|---|---|
| R1 (0%) | R2 (10%) | R3 (15%) | R4 (20%) | |
| White wheat flour, g | 200 | 180 | 170 | 160 |
| Yellow mealworm powder, g | 0 | 20 | 30 | 40 |
| Sodium bicarbonate, g | 5 | 5 | 5 | 5 |
| Sunflower oil, mL | 50 | 50 | 50 | 50 |
| Soy milk, mL | 85 | 85 | 85 | 85 |
| Honey, g | 50 | 50 | 50 | 50 |
| Vanilla sugar, g | 8 | 8 | 8 | 8 |
| Salt, g | 1 | 1 | 1 | 1 |
| Parameter | Raw Materials | Selected Biscuits | |||
|---|---|---|---|---|---|
| White Wheat Flour WWF | Yellow Mealworm Powder YMP | Reference Biscuits (100% White Wheat Flour) 0% (R1) | Biscuits Containing 15% Yellow Mealworm Powder 15% (R3) | ||
| Dry matter g/100 g | 90.27 ± 2.62 (a) | 93.52 ± 3.11 (a) | 92.34 ± 1.56 (b) | 94.98 ± 0.42 (a) | |
| Protein, g/100 g | 9.87 ± 0.29 (b) | 48.21 ± 2.20 (b) | 29.11 ± 0.46 (b) | 31.33 ± 0.53 (a) | |
| Total lipids, g/100 g | 0.90 ± 0.06 (b) | 34.21 ± 0.97 (a) | 46.43 ± 1.10 (a) | 47.81 ± 1.55 (a) | |
| Ash, g/100 g | 0.68 ± 0.03 (b) | 5.03 ± 0.26 (a) | 1.42 ± 0.09 (b) | 1.56 ± 0.04 (a) | |
| Crude fibre, g/100 g | 0.51 ± 0.02 (b) | 4.01 ± 0.13 (a) | 1.36 ± 0.12 (b) | 1.42 ± 0.05 (a) | |
| Carbohydrates, g/100 g | 88.04 ± 0.52 (a) | 8.54 ± 0.32 (b) | 21.68 ± 1.12 (a) | 17.88 ± 1.71 (b) | |
| Energy, kcal/100 g | 400.76 ± 10.30 (b) | 542.91 ± 7.54 (a) | 621.95 ± 5.72 (a) | 629.97 ± 4.36 (a) | |
| 5-HMF, mg/Kg | - | - | 6.21 ± 0.08 (a) | 4.17 ± 0.12 (b) | |
| Bioaccessible fraction of total protein (%) | - | - | 76.31 ± 3.29 (b) | 85.14 ± 4.35 (a) | |
| Bioaccessible fraction of total fat (%) | - | - | 54.07 ± 3.34 (a) | 56.38 ± 4.02 (a) | |
| Estimated Glycaemic Index, % | - | - | 90.14 ± 3.14 (a) | 79.56 ± 3.24 (b) | |
| Mineral elements, mg/100 g | Na | 180.02 ± 2.11 (b) | 265.12 ± 3.45 (a) | 381.05 ± 9.70 (b) | 389.04 ± 4.87 (a) |
| K | 123.45 ± 2.32 (b) | 865.03 ± 7.12 (a) | 386.48 ± 3.32 (b) | 413.84 ± 3.11 (a) | |
| Ca | 43.17 ± 1.67 (b) | 215.15 ± 4.83 (a) | 98.29 ± 1.67 (b) | 104.58 ± 1.86 (a) | |
| Mg | 32.45 ± 2.13 (b) | 332.16 ± 3.13 (a) | 81.33 ± 1.76 (b) | 98.12 ± 2.11 (a) | |
| P | 112.20 ± 3.02 (b) | 222.45 ± 3.20 (a) | 779.44 ± 2.45 (a) | 780.11 ± 2.33 (a) | |
| Cu | 0.11 ± 0.01 (b) | 17.46 ± 0.45 (a) | 1.77 ± 0.012 (b) | 2.47 ± 0.12 (a) | |
| Zn | 0.46 ± 0.03 (b) | 55.22 ± 2.10 (a) | 4.39 ± 0.15 (b) | 6.59 ± 0.18 (a) | |
| Mn | 0.82 ± 0.05 (b) | 13.31 ± 0.33 (a) | 2.87 ± 0.08 (b) | 3.25 ± 0.07 (a) | |
| Fe | 2.14 ± 0.11 (b) | 29.43 ± 1.76 (a) | 6.17 ± 0.21 (b) | 7.73 ± 0.12 (a) | |
| Li | 0.34 ± 0.015 (b) | 0.92 ± 0.05 (a) | 2.03 ± 0.12 (b) | 2.30 ± 0.01 (a) | |
| Fatty Acid % of Total Fatty Acids ± SD | |||||
|---|---|---|---|---|---|
| Fatty Acid | White Wheat Flour WWF | Yellow Mealworm Powder YMP | Reference Biscuits (100% White Wheat Flour) 0% (R1) | Biscuits Containing 15% Yellow Mealworm Powder 15% (R3) | |
| Caprylic | C8:0 | 0.35 ± 0.01 | Nd | 0.32 ± 0.05 (a) | 0.19 ± 0.02 (b) |
| Capric | C10:0 | 0.42 ± 0.04 | Nd | 0.25 ± 0.03 (a) | 0.23 ± 0.05 (a) |
| Lauric | C12:0 | nd | 0.21 ± 0.01 | nd | 0.03 ± 0.01 |
| Myristic | C14:0 | 0.20 ± 0.01 (b) | 1.91 ± 0.11 (a) | 0.17 ± 0.02 (b) | 0.25 ± 0.02 (a) |
| Pentadecanoic | C15:0 | 0.21 ± 0.02 (a) | 0.18 ± 0.01 (b) | 0.16 ± 0.06 (a) | 0.13 ± 0.03 (a) |
| Palmitic | C16:0 | 14.24 ± 0.05 (b) | 15.78 ± 0.22 (a) | 12.15 ± 0.027 (a) | 12.4 ± 0.21 (a) |
| Stearic | C18:0 | 1.92 ± 0.01 (b) | 2.22 ± 0.06 (a) | 2.53 ± 0.31 (a) | 2.57 ± 0.32 (a) |
| Arachidic | C20:0 | nd | 0.1 ± 0.02 | nd | 0.11 ± 0.02 |
| Behenic | C22:0 | 0.62 ± 0.03 | Nd | 0.47 ± 0.02 (a) | 0.39 ± 0.04 (b) |
| Myristoleic | C14:1, n-5 | nd | 0.06 ± 0.03 | nd | nd |
| Palmitoleic | C16:1, n-7 | 0.50 ± 0.01 (b) | 2.11 ± 0.03 (a) | 0.38 ± 0.07 (a) | 0.43 ± 0.01 (a) |
| Oleic | C18:1, n-9 | 16.65 ± 0.02 (b) | 28.13 ± 0.12 (a) | 19.3 ± 0.43 (b) | 20.14 ± 0.22 (a) |
| Gondoic | C20:1, n-9 | 0.75 ± 0.01 (a) | 0.19 ± 0.01 (b) | 0.56 ± 0.03 (a) | 0.37 ± 0.07 (b) |
| Linoleic | C18:2, n-6 | 61.47 ± 0.41 (a) | 38.90 ± 0.13 (b) | 60.11 ± 0.87 (a) | 58.72 ± 0.93 (a) |
| Linolenic | C18:3, n-3 | 2.67 ± 0.01 (b) | 10.21 ± 0.12 (a) | 3.6 ± 0.19 (b) | 4.04 ± 0.06 (a) |
| ΣSFA | 17.96 ± 0.13 (b) | 20.40 ± 0.19 (a) | 15.58 ± 0.41 (a) | 16.3 ± 0.47 (a) | |
| ΣMUFA | 17.90 ± 0.24 (b) | 30.49 ± 0.05 (a) | 20.24 ± 0.68 (a) | 20.94 ± 0.35 (a) | |
| ΣPUFA | 64.14 ± 1.52 (a) | 49.11 ± 0.34 (b) | 63.71 ± 1.52 (a) | 62.76 ± 2.54 (a) | |
| n-6/n-3 ratio | 23.02 ± 0.26 (a) | 3.81 ± 0.07 (b) | 16.70 ± 0.36 (a) | 14.53 ± 0.48 (b) | |
| PUFA/SFA ratio | 3.57 | 2.41 | 4.09 | 3.85 | |
| AI index | - | - | 0.15 | 0.16 | |
| TI index | - | - | 0.21 | 0.29 | |
| h/H index | - | - | 6.74 | 6.55 | |
| Amino Acid % of Total Amino Acids ± SD | ||||
|---|---|---|---|---|
| Amino Acid | White Wheat Flour WWF | Yellow Mealworm Powder YMP | Reference Biscuits (100% White Wheat Flour) 0% (R1) | Biscuits Containing 15% Yellow Mealworm Powder 15% (R3) |
| Essential amino acids (EAAs)% of total amino acids | ||||
| Valine | 7.34 ± 0.34 (b) | 9.11 ± 1.04 (a) | 5.02 ± 0.24 (b) | 5.97 ± 0.26 (a) |
| Leucine | 15.18 ± 0.43 (b) | 18.53 ± 0.22 (a) | 8.03 ± 0.62 (a) | 8.87 ± 0.32 (a) |
| Isoleucine | 8.04 ± 0.49 (a) | 8.83 ± 1.10 (a) | 6.34 ± 0.34 (a) | 6.35 ± 0.74 (a) |
| Methionine | 0.25 ± 0.11 (b) | 0.45 ± 0.11 (a) | 4.23 ± 0.25 (a) | 4.14 ± 0.43 (a) |
| Threonine | 1.02 ± 0.87 (a) | 1.24 ± 0.34 (a) | 4.56 ± 0.17 (a) | 5.02 ± 0362 (a) |
| Phenylalanine | 5.03 ± 0.93 (b) | 6.19 ± 0.12 (a) | 5.31 ± 0.66 (a) | 6.11 ± 0.44 (a) |
| Lysine | 0.06 ± 0.01 (b) | 0.21 ± 0.01 (a) | 4.46 ± 0.57 (a) | 5.21 ± 0.34 (a) |
| Histidine | nd | nd | nd | nd |
| Tyrosine | 2.53 ± 1.03 (b) | 11.92 ± 0.64 (a) | 7.12 ± 0.90 (a) | 8.64 ± 1.92 (a) |
| Total EAAs | 39.45 ± 0.41 (b) | 56.48 ± 3.59 (a) | 45.07 ± 087 (b) | 50.31 ± 1.14 (a) |
| Nonessential amino acids (NEAAs)% of total amino acids | ||||
| Alanine | 6.32 ± 1.12 (b) | 7.78 ± 0.09 (a) | 4.39 ± 0.17 (a) | 4.34 ± 0.21 (a) |
| Glycine | 2.68 ± 0.22 (a) | 3.35 ± 0.59 (a) | 3.32 ± 0.08 (b) | 3.76 ± 0.34 (a) |
| Proline | 14.06 ± 0.21 (a) | 5.01 ± 0.43 (b) | 21.27 ± 0.74 (a) | 17.11 ± 0.77 (b) |
| Serine | 3.44 ± 1.27 (a) | 2.12 ± 0.87 (a) | 3.82 ± 0.31 (a) | 3.82 ± 0.26 (a) |
| Aspartic acid | 2.82 ± 0.10 (a) | 3.67 ± 0.96 (a) | 1.85 ± 0.34 (a) | 1.83 ± 0.08 (a) |
| Hydroxyproline | 0.02 ± 0.01 (b) | 0.12 ± 0.01 (a) | nd | nd |
| Glutamic acid | 28.01 ± 0.02 (a) | 4.75 ± 1.34 (b) | 14.4 ± 0.65 (a) | 11.22 ± 0.18 (b) |
| Asparagine | nd | nd | 1.86 ± 0.07 (a) | 1.52 ± 0.03 (b) |
| Glutamine | nd | nd | nd | nd |
| Arginine | 3.20 ± 0.16 (b) | 16.72 ± 0.44 (a) | 4.02 ± 0.09 (b) | 6.09 ± 0.04 (a) |
| Total NEAAs | 60.55 ± 0.28 (a) | 43.52 ± 5.72 (b) | 54.93 ± 1.48 (a) | 49.69 ± 0.11 (b) |
| LOS Model | Black Line in Figure 2b | Blue Line in Figure 2b | Red Line in Figure 2b |
|---|---|---|---|
| 1. The linear regression model | |||
| n | 21 | 6 | 15 |
| a | 0.5192 | 2.0225 | 0.1061 |
| b | −0.0331 | −0.1262 | −0.0296 |
| R | −0.9090 | −0.9798 | −0.8422 |
| R2 | 0.8263 | 0.9600 | 0.7094 |
| Adj R2 | 0.8171 | 0.9499 | 0.6870 |
| SEE | 0.8762 | 0.2696 | 0.8798 |
| 2. Analysis of variance | |||
| SS reg | 69.3702 | 6.9688 | 24.5623 |
| df reg | 1 | 1 | 1 |
| MS reg | 69.3702 | 6.9688 | 24.5623 |
| SS error | 14.5874 | 0.2907 | 10.0625 |
| df error | 19 | 4 | 13 |
| MS error | 0.7678 | 0.0727 | 0.7740 |
| F | 90.3543 | 95.8752 | 31.7326 |
| p | 1.1837 × 10 −8 | 6.0972 × 10 −4 | 8.1570 × 10 −5 |
| 3. Statistics of regression model coefficients | |||
| Sy/x | 0.8762 | 0.2696 | 0.8798 |
| Sa | 0.3361 | 46.5344 | 0.5970 |
| ta | 1.5445 | 0.0435 | 0.1777 |
| pa | 0.1390 | 0.9674 | 0.8617 |
| Sb | 0.0035 | 2.6960 | 0.0053 |
| tb | −9.5055 | −0.0468 | −5.6332 |
| pb | 1.1837 × 10 −8 | 9.6491 × 10 −1 | 8.1570 × 10 −5 |
| 4. The coordinates of the intersection point | |||
| x [min] | y [ln(%/min)] | ||
| [d1] ∩ [d2] | 19.8411 | 2.0162 | |
| Reference Biscuits (100% White Wheat Flour) 0% (R1) | Biscuits Containing 15% Yellow Mealworm Powder 15% (R3) | |
|---|---|---|
| LOS model | ||
| KRDS, min−1 | 0.12 ± 0.01 (a) | 0.09 ± 0.01 (b) |
| KSDS, min−1 | 0.02 ± 0.01 (a) | 0.01 ± 0.00 (a) |
| ts, min | 28.29 ± 7.40 (a) | 25.99 ± 4.37 (a) |
| NLLS model | ||
| CRDS∞, % | 61.02 ± 2.84 (a) | 45.73 ± 2.19 (b) |
| CSDS∞, % | 21.15 ± 5.35 (b) | 32.45 ± 0.83 (a) |
| KRDS, min−1 | 0.14 ± 0.01 (a) | 0.13 ± 0.01 (a) |
| KSDS, min−1 | 0.01 ± 0.01 (a) | 0.01 ± 0.00 (a) |
| ts, min | 26.71 ± 7.01 (a) | 22.87 ± 0.56 (a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaly Cozmuta, A.; Uivarasan, A.; Peter, A.; Nicula, C.; Kovacs, D.E.; Mihaly Cozmuta, L. Yellow Mealworm (Tenebrio molitor) Powder Promotes a High Bioaccessible Protein Fraction and Low Glycaemic Index in Biscuits. Nutrients 2023, 15, 997. https://doi.org/10.3390/nu15040997
Mihaly Cozmuta A, Uivarasan A, Peter A, Nicula C, Kovacs DE, Mihaly Cozmuta L. Yellow Mealworm (Tenebrio molitor) Powder Promotes a High Bioaccessible Protein Fraction and Low Glycaemic Index in Biscuits. Nutrients. 2023; 15(4):997. https://doi.org/10.3390/nu15040997
Chicago/Turabian StyleMihaly Cozmuta, Anca, Alexandra Uivarasan, Anca Peter, Camelia Nicula, Dalma Emoke Kovacs, and Leonard Mihaly Cozmuta. 2023. "Yellow Mealworm (Tenebrio molitor) Powder Promotes a High Bioaccessible Protein Fraction and Low Glycaemic Index in Biscuits" Nutrients 15, no. 4: 997. https://doi.org/10.3390/nu15040997
APA StyleMihaly Cozmuta, A., Uivarasan, A., Peter, A., Nicula, C., Kovacs, D. E., & Mihaly Cozmuta, L. (2023). Yellow Mealworm (Tenebrio molitor) Powder Promotes a High Bioaccessible Protein Fraction and Low Glycaemic Index in Biscuits. Nutrients, 15(4), 997. https://doi.org/10.3390/nu15040997