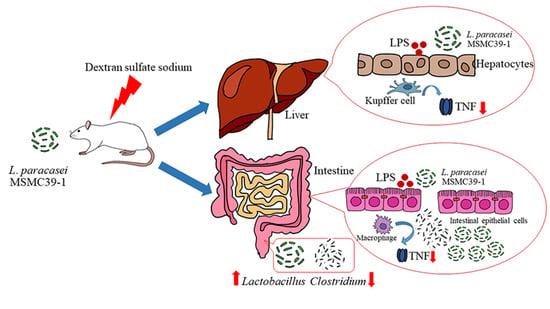
Anti-Inflammatory and Gut Microbiota Modulating Effects of Probiotic Lactobacillus paracasei MSMC39-1 on Dextran Sulfate Sodium-Induced Colitis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Culture Supernatant and Viable Cells Preparation
2.2. Heat-Killed Cells Preparation
2.3. Sonicated Cells Preparation
2.4. Caco-2 Cells
2.5. The effect of Probiotic Strains in the TNF-α Production in Caco-2 Cells
2.6. Animal Experiment
2.7. Induction of Colitis
2.8. Experimental Designs
2.9. Detection of Aspartate Aminotransferase and Alanine Aminotransferase
2.10. Detection of TNF-α in the Colon and Hepatic Tissue
2.11. Histology Evaluation of Colitis Rats
2.12. Microbiota Detection using 16S rDNA Next-Generation Sequencing (NGS)
2.13. Statistical Analysis
3. Results
3.1. The effect of Probiotic Strains on TNF-α Production in Caco-2 Cells
3.2. Effect of Probiotic L. paracasei Strain MSMC39-1 on Body Weight and Stool Consistency in DSS-Induced Colitis Rats
3.3. Effect of Probiotic L. paracasei Strain MSMC39-1 on Liver Functional Enzyme Activity in DSS-Induced Colitis Rats
3.4. Effect of Probiotic L. paracasei Strain MSMC39-1 on TNF-α Production in Colon and Liver Tissues of DSS-Induced Colitis Rats
3.5. Effect of Probiotic L. paracasei Strain MSMC39-1 on Colon and Liver Tissue Histology in DSS-Induced Colitis Rats
3.6. Effect of L. paracasei Strain MSMC39-1 on Microbiota Modulation in DSS-Induced Colitis Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Segal, J.P.; LeBlanc, J.F.; Hart, A.L. Ulcerative colitis: An update. Clin. Med. 2021, 21, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A comprehensive review and update on the pathogenesis of Inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Liang, D.; Leung, R.K.; Guan, W.; Au, W.W. Involvement of gut microbiome in human health and disease: Brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018, 10, 3. [Google Scholar] [CrossRef]
- Chu, W.-M. Tumor Necrosis Factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Lu, Y.C.; Ou, C.C.; Lin, S.L.; Tsai, C.C.; Huang, C.T.; Lin, M.Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ho, Y.F.; Chen, Y.P.; Chen, M.J. Effects of a novel encapsulating technique on the temperature tolerance and anti-colitis activity of the probiotic bacterium Lactobacillus kefiranofaciens M1. Food Microbiol. 2015, 46, 494–500. [Google Scholar] [CrossRef]
- Lim, S.M.; Jang, H.M.; Jeong, J.J.; Han, M.J.; Kim, D.H. Lactobacillus johnsonii CJLJ103 attenuates colitis and memory impairment in mice by inhibiting gut microbiota lipopolysaccharide production and NF-κB activation. J. Funct. Foods 2017, 34, 359–368. [Google Scholar] [CrossRef]
- Seo, S.; Shin, J.-S.; Lee, W.-S.; Rhee, Y.K.; Cho, C.-W.; Hong, H.-D.; Lee, K.-T. Anti-colitis effect of Lactobacillus sakei K040706 via suppression of inflammatory responses in the dextran sulfate sodium-induced colitis mice model. J. Funct. Foods 2017, 29, 256–268. [Google Scholar] [CrossRef]
- Stofilova, J.; Langerholc, T.; Botta, C.; Treven, P.; Gradisnik, L.; Salaj, R.; Soltesova, A.; Bertkova, I.; Hertelyova, Z.; Bomba, A. Cytokine production in vitro and in rat model of colitis in response to Lactobacillus plantarum LS/07. Biomed. Pharmacother. 2017, 94, 1176–1185. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsu, P.Y.; Pan, T.M. Therapeutic effects of Lactobacillus paracasei subsp. paracasei NTU 101 powder on dextran sulfate sodium-induced colitis in mice. J. Food Drug Anal. 2019, 27, 83–92. [Google Scholar] [CrossRef]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.-S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. The effects of orally administered Bifidobacterium animalis subsp. lactis strain BB12 on dextran sodium sulfate-induced colitis in mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Polk, D.B.; Tomasula, P.M.; Yan, F.; Liu, L.S. Preserving viability of Lactobacillus rhamnosus GG in vitro and in vivo by a new encapsulation system. J. Control. Release 2016, 230, 79–87. [Google Scholar] [CrossRef]
- Kim, Y.; Koh, J.H.; Ahn, Y.J.; Oh, S.; Kim, S.H. The synergic anti-inflammatory impact of Gleditsia sinensis Lam. and Lactobacillus brevis KY21 on intestinal epithelial cells in a DSS-induced colitis model. Korean J. Food Sci. Anim. Resour. 2015, 35, 604–610. [Google Scholar] [CrossRef]
- Jo, S.G.; Noh, E.J.; Lee, J.Y.; Kim, G.; Choi, J.H.; Lee, M.E.; Song, J.H.; Chang, J.Y.; Park, J.H. Lactobacillus curvatus WiKim38 isolated from kimchi induces IL-10 production in dendritic cells and alleviates DSS-induced colitis in mice. J. Microbiol. 2016, 54, 503–509. [Google Scholar] [CrossRef]
- Gholami, M.; Ghasemi-Niri, S.F.; Maqbool, F.; Baeeri, M.; Memariani, Z.; Pousti, I.; Abdollahi, M. Experimental and pathalogical study of Pistacia atlantica, butyrate, Lactobacillus casei and their combination on rat ulcerative colitis model. Pathol. Res. Pract. 2016, 212, 500–508. [Google Scholar] [CrossRef]
- Kondo, S.; Kuda, T.; Nemoto, M.; Usami, Y.; Takahashi, H.; Kimura, B. Protective effects of rice bran fermented by Saccharomyces cerevisiae Misaki-1 and Lactobacillus plantarum Sanriki-SU8 in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci. 2016, 16, 44–49. [Google Scholar] [CrossRef]
- Jang, S.-H.; Park, J.; Kim, S.-H.; Choi, K.-M.; Ko, E.-S.; Cha, J.-D.; Lee, Y.-R.; Jang, H.; Jang, Y.-S. Oral administration of red ginseng powder fermented with probiotic alleviates the severity of dextran-sulfate sodium-induced colitis in a mouse model. Chin. J. Nat. Med. 2017, 15, 192–201. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Bedani, R.; Dejani, N.N.; Ivo de Medeiros, A.; Sampaio Zuanon, J.A.; Spolidorio, L.C.; Tallarico Adorno, M.A.; Amancio Varesche, M.B.; Carrilho Galvao, F.; Valentini, S.R.; et al. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. PLoS ONE 2017, 12, e0175935. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, X.; Wang, H.; Yang, Z.; Li, J.; Suo, H. Prevent Effects of Lactobacillus fermentum HY01 on Dextran sulfate sodium-induced colitis in mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Bosco, N.; Perruisseau, G.; Nicolas, J.; Segura-Roggero, I.; Duboux, S.; Briand, M.; Blum, S.; Benyacoub, J. Lactobacillus paracasei reduces intestinal inflammation in adoptive transfer mouse model of experimental colitis. Clin. Dev. Immunol. 2011, 2011, 807483. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Mattace Raso, G.; Lama, A.; Pirozzi, C.; Santoro, A.; Di Guida, F.; Sanges, M.; Aksoy, E.; Calignano, A.; D’Arienzo, A.; et al. Preventive and therapeutic effects of Lactobacillus paracasei B21060-based synbiotic treatment on gut inflammation and barrier integrity in colitic mice. J. Nutr. 2015, 145, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Ladda, B.; Theparee, T.; Chimchang, J.; Tanasupawat, S.; Taweechotipatr, M. In vitro modulation of tumor necrosis factor alpha production in THP-1 cells by lactic acid bacteria isolated from healthy human infants. Anaerobe 2015, 33, 109–116. [Google Scholar] [CrossRef]
- Jantararussamee, C.; Rodniem, S.; Taweechotipatr, M.; Showpittapornchai, U.; Pradidarcheep, W. Hepatoprotective effect of probiotic lactic acid bacteria on Thioacetamide-induced liver fibrosis in rats. Probiotics Antimicrob. Proteins 2021, 13, 40–50. [Google Scholar] [CrossRef]
- Ladda, B.; Tangteerawatana, P.; Padungchaichot, P.; Pradidarcheep, W.; Kasorn, A.; Taweechotipatr, M. Anti-inflammatory effect of probiotic Lactobacillus paracasei MSMC39-1 on alcohol-induced hepatitis in rats. J. Appl. Pharm. Sci. 2021, 11, 46–56. [Google Scholar] [CrossRef]
- Banjonjit, S.; Taweechotipatr, M.; Rungsiyanont, S. Effect of probiotic Lactobacillus paracasei on tumor necrosis factor-alpha level in gingival crevicular fluid of patients undergoing impacted third molar removal. J. Oral Sci. 2022, 64, 185–189. [Google Scholar] [CrossRef]
- Sathikulpakdee, S.; Kanokrungsee, S.; Vitheejongjaroen, P.; Kamanamool, N.; Udompataikul, M.; Taweechotipatr, M. Efficacy of probiotic-derived lotion from Lactobacillus paracasei MSMC 39-1 in mild to moderate acne vulgaris, randomized controlled trial. J. Cosmet. Dermatol. 2022, 21, 5092–5097. [Google Scholar] [CrossRef]
- Morin, C.; Blier, P.U.; Fortin, S. MAG-EPA reduces severity of DSS-induced colitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G808–G821. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Y.; Ni, Y.; Zhang, T.; Duan, C.; Huang, C.; Zhao, Y.; Gao, L.; Li, S. Protective effects of Lactobacillus plantarum C88 on chronic ethanol-induced liver injury in mice. J. Funct. Foods 2017, 35, 97–104. [Google Scholar] [CrossRef]
- Food and Agricultural Organization and the World Health Organization. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Jeong, J.J.; Woo, J.Y.; Ahn, Y.T.; Shim, J.H.; Huh, C.S.; Im, S.H.; Han, M.J.; Kim, D.H. The probiotic mixture IRT5 ameliorates age-dependent colitis in rats. Int. Immunopharmacol. 2015, 26, 416–422. [Google Scholar] [CrossRef]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Vieira, A.T.; Rocha, V.M.; Tavares, L.; Garcia, C.C.; Teixeira, M.M.; Oliveira, S.C.; Cassali, G.D.; Gamba, C.; Martins, F.S.; Nicoli, J.R. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 5(1A). Microbes Infect 2016, 18, 180–189. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Kessoku, T.; Kobayashi, T.; Imajo, K.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Kasai, Y.; Ozaki, A.; Iwaki, M.; Nogami, A.; et al. Endotoxins and non-alcoholic fatty liver disease. Front. Endocrinol. 2021, 12, 770986. [Google Scholar] [CrossRef]
- Nascimento, R.P.D.; Machado, A.; Galvez, J.; Cazarin, C.B.B.; Marostica Junior, M.R. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef]
- Sepulveda, J. Chapter 9—Challenges in routine clinical chemistry analysis: Proteins and enzymes. In Accurate Results in the Clinical Laboratory; Dasgupta, A., Sepulveda, J.L., Eds.; Elsevier: San Diego, CA, USA, 2013; pp. 131–148. [Google Scholar]
- Jang, S.E.; Jeong, J.J.; Kim, J.K.; Han, M.J.; Kim, D.H. Simultaneous amelioratation of colitis and liver injury in mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci. Rep. 2018, 8, 7500. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Gao, H.; Wang, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. Bifidobacterium breve CCFM683 could ameliorate DSS-induced colitis in mice primarily via conjugated linoleic acid production and gut microbiota modulation. J. Funct. Foods 2018, 49, 61–72. [Google Scholar] [CrossRef]
- Jin, J.; Wu, S.; Xie, Y.; Liu, H.; Gao, X.; Zhang, H. Live and heat-killed cells of Lactobacillus plantarum Zhang-LL ease symptoms of chronic ulcerative colitis induced by dextran sulfate sodium in rats. J. Funct. Foods 2020, 71, 103994. [Google Scholar] [CrossRef]
- Wang, G.; Tang, H.; Zhang, Y.; Xiao, X.; Xia, Y.; Ai, L. The intervention effects of Lactobacillus casei LC2W on Escherichia coli O157:H7 -induced mouse colitis. Food Sci. Hum. Wellness 2020, 9, 289–294. [Google Scholar] [CrossRef]
- Wasilewska, E.; Zlotkowska, D.; Wroblewska, B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. J. Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Koh, J.H.; Park, M.R.; Ryu, S.; Lee, W.J.; Yun, B.; Lee, J.H.; Oh, S.; Kim, Y. Effect of the Lactobacillus rhamnosus strain GG and tagatose as a synbiotic combination in a dextran sulfate sodium-induced colitis murine model. J. Dairy Sci. 2019, 102, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Zhang, J.; Mu, J.; Zalan, Z.; Hegyi, F.; Takacs, K.; Zhao, X.; Du, M. Protective effect of Lactobacillus fermentum CQPC04 on dextran sulfate sodium-induced colitis in mice is associated with modulation of the nuclear factor-kappaB signaling pathway. J. Dairy Sci. 2019, 102, 9570–9585. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Lin, J.; Xing, Y.-F.; Zeng, W.; Zhu, W.-M.; Su, N.; Zhang, C.; Lu, Y.; Xing, X.-H. Identification and characterization of alcohol-soluble components from wheat germ-apple fermented by Lactobacillus sp. capable of preventing ulcerative colitis of dextran sodium sulfate-induced mice. J. Funct. Foods 2020, 64, 103642. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.; Ahn, J.B.; Kim, J.H.; Ma, H.W.; Seo, D.H.; Che, X.; Park, K.C.; Jeon, J.Y.; Kim, S.Y.; et al. Lactobacillus plantarum CBT LP3 ameliorates colitis via modulating T cells in mice. Int. J. Med. Microbiol. 2020, 310, 151391. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Hong, G.; Huang, C.; Qian, W.; Bai, T.; Song, J.; Song, Y.; Hou, X. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci. 2020, 240, 117089. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Y.; Stanton, C.; Ross, R.P.; Wang, Z.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Dose-response efficacy and mechanisms of orally administered CLA-producing Bifidobacterium breve CCFM683 on DSS-induced colitis in mice. J. Funct. Foods 2020, 75, 104245. [Google Scholar] [CrossRef]
- Somineni, H.K.; Kugathasan, S. The microbiome in patients with inflammatory diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 243–255. [Google Scholar] [CrossRef]








| Score | Characteristics |
|---|---|
| 0 | no ulcer and inflammation |
| 1 | no ulcer and local hyperemia |
| 2 | ulcer without hyperemia |
| 3 | ulcer and one site only of inflammation |
| 4 | two or more sites of ulcer and inflammation |
| 5 | ulcer extending more than 2 cm |
| Group | ||||
|---|---|---|---|---|
| Parameters | Negative Control | Probiotic Control | Colitis Control | Probiotic Test |
| Body weight increase (g) | 125.00 ± 9.98 | 122.50 ± 23.91 | 93.41 ± 6.80 | 107.40 ± 11.03 |
| Stool consistency | Formed | Formed | Loose, bloody | Formed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladda, B.; Jantararussamee, C.; Pradidarcheep, W.; Kasorn, A.; Matsathit, U.; Taweechotipatr, M. Anti-Inflammatory and Gut Microbiota Modulating Effects of Probiotic Lactobacillus paracasei MSMC39-1 on Dextran Sulfate Sodium-Induced Colitis in Rats. Nutrients 2023, 15, 1388. https://doi.org/10.3390/nu15061388
Ladda B, Jantararussamee C, Pradidarcheep W, Kasorn A, Matsathit U, Taweechotipatr M. Anti-Inflammatory and Gut Microbiota Modulating Effects of Probiotic Lactobacillus paracasei MSMC39-1 on Dextran Sulfate Sodium-Induced Colitis in Rats. Nutrients. 2023; 15(6):1388. https://doi.org/10.3390/nu15061388
Chicago/Turabian StyleLadda, Boonyarut, Chittapon Jantararussamee, Wisuit Pradidarcheep, Anongnard Kasorn, Udomlak Matsathit, and Malai Taweechotipatr. 2023. "Anti-Inflammatory and Gut Microbiota Modulating Effects of Probiotic Lactobacillus paracasei MSMC39-1 on Dextran Sulfate Sodium-Induced Colitis in Rats" Nutrients 15, no. 6: 1388. https://doi.org/10.3390/nu15061388
APA StyleLadda, B., Jantararussamee, C., Pradidarcheep, W., Kasorn, A., Matsathit, U., & Taweechotipatr, M. (2023). Anti-Inflammatory and Gut Microbiota Modulating Effects of Probiotic Lactobacillus paracasei MSMC39-1 on Dextran Sulfate Sodium-Induced Colitis in Rats. Nutrients, 15(6), 1388. https://doi.org/10.3390/nu15061388