Early Intervention of Elateriospermum tapos Yoghurt in Obese Dams Mitigates Intergenerational Cognitive Deficits and Thigmotactic Behaviour in Male Offspring via the Modulation of Metabolic Profile
Abstract
:1. Introduction
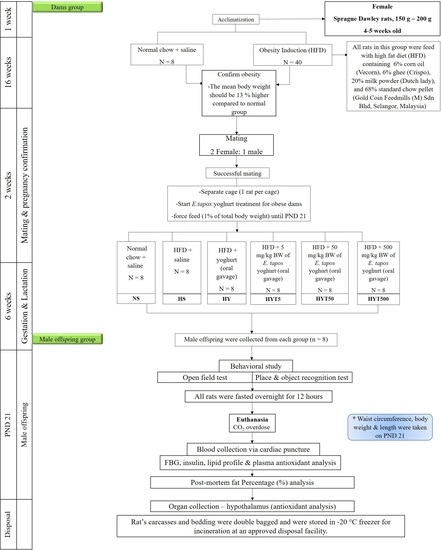
2. Materials and Methods
2.1. Collection and Confirmation of E. tapos Seed
2.2. Ethanol Extraction of E. tapos Seed
2.3. Formulation of E. tapos Yoghurt
2.4. High-Fat Diet Preparation
2.5. Experimental Animals
2.6. Obesity Induction
2.7. Mating, Gestation, and Weaning
2.8. Anthropometrical Determinations
2.9. Anxiety Test
2.10. Novel Object Recognition Test (NORT) and Place Recognition Test (PRT)
2.11. Fasting Blood Glucose Level
2.12. Postmortem Fat Percentage (%) Analysis
2.13. Insulin Level
2.14. Lipid Profile
2.15. Antioxidant Parameter
2.16. Statistical Analysis
3. Results
3.1. Body Mass Index (BMI), Lee Index, and Abdominal Circumference of Male Offspring on PND 21
3.2. Fasting Blood Glucose in Male Offspring on PND 21
3.3. Anxiety Test in Male Offspring on PND 21
3.4. Novel Object Recognition Test (NORT) and Place Recognition Test (PRT) in Male Offspring on PND 21
3.5. Fat Percentage (%) in Male Offspring on PND 21
3.6. Lipid Profile of Male Offspring on PND 21
3.7. Insulin Level in Male Offspring on PND 21
3.8. Antioxidants Level in Serum and Hypothalamus of Male Offspring on PND 21
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2018, 37, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Dabelea, D.; Crume, T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011, 60, 1849–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forhead, A.J.; Fowden, A.L. The hungry fetus? Role of leptin as a nutritional signal before birth. J. Physiol. 2008, 587, 1145. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Riper, K.M.; Lockard, R.; Valleau, J.C. Maternal High-Fat Diet Programming of the Neuroendocrine System and Behavior. Horm. Behav. 2015, 76, 153. [Google Scholar] [CrossRef] [Green Version]
- Hasebe, K.; Kendig, M.D.; Morris, M.J. Mechanisms underlying the cognitive and behavioural effects of maternal obesity. Nutrients 2021, 13, 240. [Google Scholar] [CrossRef]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between Peripheral and Central Inflammation in Obesity-Promoted Disorders: The Impact on Synaptic Mitochondrial Functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef]
- Penna, E.; Pizzella, A.; Cimmino, F.; Trinchese, G.; Cavaliere, G.; Catapano, A.; Allocca, I.; Chun, J.T.; Campanozzi, A.; Messina, G.; et al. Neurodevelopmental Disorders: Effect of High-Fat Diet on Synaptic Plasticity and Mitochondrial Functions. Brain Sci. 2020, 10, 805. [Google Scholar] [CrossRef]
- Chan, J.Y.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Messina, G.; Monda, M.; Crispino, M.; Mollica, M.P. Long Feeding High-Fat Diet Induces Hypothalamic Oxidative Stress and Inflammation, and Prolonged Hypothalamic AMPK Activation in Rat Animal Model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef] [Green Version]
- Adenan, D.M.; Jaafar, Z.; Jayapalan, J.J.; Aziz, A.A. Plasma antioxidants and oxidative stress status in obese women: Correlation with cardiopulmonary response. PeerJ 2019, 8, e9230. [Google Scholar] [CrossRef]
- Fabianová, K.; Babeľová, J.; Fabian, D.; Popovičová, A.; Martončíková, M.; Raček, A.; Račeková, E. Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 5564. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Milazzo, R.; Savasi, V.M.; Cetin, I. Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. Int. J. Mol. Sci. 2021, 22, 1732. [Google Scholar] [CrossRef]
- Freeman, L.R.; Haley-Zitlin, V.; Rosenberger, D.S.; Granholm, A.C. Damaging effects of a high-fat diet to the brain and cognition: A review of proposed mechanisms. Nutr. Neurosci. 2014, 17, 241. [Google Scholar] [CrossRef]
- Peral-Sanchez, I.; Hojeij, B.; Ojeda, D.A.; Steegers-Theunissen, R.P.M.; Willaime-Morawek, S. Epigenetics in the Uterine Environment: How Maternal Diet and ART May Influence the Epigenome in the Offspring with Long-Term Health Consequences. Genes 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The impact of maternal high-fat diet on offspring neurodevelopment. Front. Neurosci. 2022, 16, 1148. [Google Scholar] [CrossRef]
- Shook, L.L.; Kislal, S.; Edlow, A.G. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 2020, 40, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Usall, J.; Cobo, J.; Labad, X.; Kulkarni, J. Gender Differences in Schizophrenia and First-Episode Psychosis: A Comprehensive Literature Review. Schizophr. Res. Treat. 2012, 2012, 91698. [Google Scholar] [CrossRef] [Green Version]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT-Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.R.; Martel, F. Sibutramine Effects on Central Mechanisms Regulating Energy Homeostasis. Curr. Neuropharmacol. 2011, 10, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Perumal, K.V.; Ja’afar, N.L.; Taib, C.N.M.; Shafie, N.H.; Bahari, H. Antiobesity activity of elateriospermum tapos shell extract in obesity-induced sprague dawley rats. Molecules 2021, 26, 321. [Google Scholar] [CrossRef] [PubMed]
- Abidin, A.Z.; Balan, S.S.; Perumal, K.V.; Shafie, N.H.; Abdullah, M.A.; Jasni, A.S.; Kadir, K.K.A.; Bahari, H. Elateriospermum tapos Supplementation in Dams Ameliorating Obesity Development and Stress Hormone Level among Adult Male Offspring. Proceedings 2020, 61, 2. [Google Scholar] [CrossRef]
- Naomi, R.; Nabila, R.; Rusli, M.; Othman, F.; Segaran Balan, S.; Abidin, A.Z.; Embong, H.; Teoh, S.H.; Jasni, A.S.; Jumidil, S.H.; et al. Elateriospermum tapos Yogurt Supplement in Maternal Obese Dams during Pregnancy Modulates the Body Composition of F1 Generation. Nutrients 2023, 15, 1258. [Google Scholar] [CrossRef]
- Lim, T.K. Elateriospermum tapos. In Edible Medicinal And Non-Medicinal Plants; Springer: Dordrecht, Germany, 2012; Volume 2, pp. 1–4. [Google Scholar] [CrossRef]
- Panahi, S.; Gallant, A.; Tremblay, A.; Pérusse, L.; Després, J.P.; Drapeau, V. The relationship between yogurt consumption, body weight, and metabolic profiles in youth with a familial predisposition to obesity. Eur. J. Clin. Nutr. 2018, 73, 541–548. [Google Scholar] [CrossRef]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: A randomized, double-blind, placebo-controlled trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Naomi, R.; Rusli, R.N.M.; Balan, S.S.; Othman, F.; Jasni, A.S.; Jumidil, S.H.; Bahari, H.; Yazid, M.D.E. tapos Yoghurt—A View from Nutritional Composition and Toxicological Evaluation. Foods 2022, 11, 1903. [Google Scholar] [CrossRef]
- Holkem, A.T.; da Silva, M.P.; Favaro-Trindade, C.S. Probiotics and plant extracts: A promising synergy and delivery systems. Crit. Rev. Food Sci. Nutr. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Yang, C.; Jiang, S.; Yang, W.; Jiang, S. The Combination of Plant Extracts and Probiotics Improved Jejunal Barrier and Absorption Capacity of Weaned Piglets. Agriculture 2022, 12, 912. [Google Scholar] [CrossRef]
- Shams Shargh, M.; Dastar, B.; Zerehdaran, S.; Khomeiri, M.; Moradi, A. Effects of using plant extracts and a probiotic on performance, intestinal morphology, and microflora population in broilers. J. Appl. Poult. Res. 2010, 21, 201–208. [Google Scholar] [CrossRef]
- Siccama, J.W.; Pegiou, E.; Zhang, L.; Mumm, R.; Hall, R.D.; Boom, R.M.; Schutyser, M.A.I. Maltodextrin improves physical properties and volatile compound retention of spray-dried asparagus concentrate. LWT-Food Sci. Technol. 2021, 142, 111058. [Google Scholar] [CrossRef]
- Tisadondilok, S.; Senawong, T.; Swatsitang, P.; Rattanasing, A. Antioxidant and antiproliferative activities of ethanolic extracts of Elateriospermum tapos Blume (Euphorbiaceae). J. Med. Plants Res. 2018, 12, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Aril-Dela Cruz, J.V.; Bungihan, M.E.; Dela Cruz, T.E.E.; Sagum, R.S. Canarium ovatum engl. (Pili) exocarp crude extract as functional food colorant incorporated in yogurt developed product. Food Res. 2017, 2, 89–98. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R46–R54. [Google Scholar] [CrossRef] [Green Version]
- Bahari, H.; Abidin, A.Z.; Balan, S.S.; Perumal, K.V.; Rosli, N.S.; Lotafi, A.H.A.; Danabalan, S.; Manimaran, M.; Shafie, N.H.; Abdullah, M.A.; et al. The effects of Elateriospermum tapos against obese maternal rat in mitigating obesity development among their adult female offspring. Pharmacogn. Mag. 2020, 16, 706–712. [Google Scholar] [CrossRef]
- Kadir, N.A.A.; Rahmat, A.; Jaafar, H.Z.E. Protective Effects of Tamarillo Extract Against High Fat Diet Induced Obesity In Sprague Dawley Rats. J. Obes. 2015, 2015, 846041. [Google Scholar] [CrossRef] [Green Version]
- Ypsilantis, P.; Somalou, P.; Panidou, E.; Simopoulos, C. Laparoscopic early pregnancy diagnosis in the laboratory rat. Lab. Anim. 2018, 52, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Athesh, K.; Agnel Arul John, N.; Sridharan, G.; Brindha, P.; Alanazi, A.M.; Rengasamy, K.R.R.; Balamuralikrishnan, B.; Liu, W.C.; Vijaya Anand, A. Protective Effect of Dolichos biflorus Seed Extract on 3T3-L1 Preadipocyte Differentiation and High-Fat Diet-Induced Obesity in Rats. Evid. Based Complement. Altern. Med. 2023, 2023, 6251200. [Google Scholar] [CrossRef]
- Timotius, I.K.; Moceri, S.; Plank, A.C.; Habermeyer, J.; Canneva, F.; Winkler, J.; Klucken, J.; Casadei, N.; Riess, O.; Eskofier, B.; et al. Silhouette-length-scaled gait parameters for motor functional analysis in mice and rats. eNeuro 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Bastías-Pérez, M.; Serra, D.; Herrero, L. Dietary options for rodents in the study of obesity. Nutrients 2020, 12, 3234. [Google Scholar] [CrossRef]
- Kuniishi, H.; Ichisaka, S.; Yamamoto, M.; Ikubo, N.; Matsuda, S.; Futora, E.; Harada, R.; Ishihara, K.; Hata, Y. Early deprivation increases high-leaning behavior, a novel anxiety-like behavior, in the open field test in rats. Neurosci. Res. 2017, 123, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 2016, 306, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Qinna, N.A.; Badwan, A.A. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des. Devel. Ther. 2015, 9, 2515–2525. [Google Scholar] [CrossRef] [Green Version]
- Tekus, E.; Miko, A.; Furedi, N.; Rostas, I.; Tenk, J.; Kiss, T.; Szitter, I.; Balasko, M.; Helyes, Z.; Wilhelm, M.; et al. Body fat of rats of different age groups and nutritional states: Assessment by micro-CT and skinfold thickness. J. Appl. Physiol. 2016, 124, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Gheni, G.; Yokoi, N.; Beppu, M.; Yamaguchi, T.; Hidaka, S.; Kawabata, A.; Hoshino, Y.; Hoshino, M.; Seino, S. Characterization of the prediabetic state in a novel rat model of type 2 diabetes, the ZFDM rat. J. Diabetes Res. 2015, 2015, 261418. [Google Scholar] [CrossRef] [Green Version]
- Aberare, O.L.; Okuonghae, P.; Mukoro, N.; Dirisu, J.O.; Osazuwa, F.; Odigie, E.; Omoregie, R. Triglycerides, total cholesterol, high density lipoprotein cholesterol and low density lipoprotein cholesterol in rats exposed to premium motor spirit fumes. N. Am. J. Med. Sci. 2011, 3, 277–280. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6940515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.S.; Naqvi, S.; Gupta, S.K.; Srivastava, S. Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms. Food Chem. Toxicol. 2012, 50, 3126–3132. [Google Scholar] [CrossRef]
- Tajaddini, A.; Kendig, M.D.; Prates, K.V.; Frederick Westbrook, R.; Morris, M.J. Male Rat Offspring Are More Impacted by Maternal Obesity Induced by Cafeteria Diet Than Females—Additive Effect of Postweaning Diet. Int. J. Mol. Sci. 2022, 23, 1442. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Nilsson, C.; Rosendal, A.; Nielsen, M.O.; Raun, K. Maternal chocolate and sucrose soft drink intake induces hepatic steatosis in rat offspring associated with altered lipid gene expression profile. Acta Physiol. 2014, 210, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Jo, H.; Schieve, L.A.; Sharma, A.J.; Hinkle, S.N.; Li, R.; Lind, J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 2014, 135, e1198–e1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. Int. J. Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, J.R.; Reynolds, R.M. The risk of maternal obesity to the long-term health of the offspring. Clin. Endocrinol. 2013, 78, 9–16. [Google Scholar] [CrossRef]
- Oken, E.; Thompson, J.W.; Rifas-Shiman, S.L.; Vilchuk, K.; Bogdanovich, N.; Hameza, M.; Yang, S.; Patel, R.; Kramer, M.S.; Martin, R.M. Analysis of Maternal Prenatal Weight and Offspring Cognition and Behavior: Results from the Promotion of Breastfeeding Intervention Trial (PROBIT) Cohort. JAMA Netw. Open 2021, 4, e2121429. [Google Scholar] [CrossRef]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef]
- Lof, J.; Smits, K.; Melotte, V.; Kuil, L.E. The health effect of probiotics on high-fat diet-induced cognitive impairment, depression and anxiety: A cross-species systematic review. Neurosci. Biobehav. Rev. 2022, 136, 104634. [Google Scholar] [CrossRef]
- Wiedmer, E.B.; Herter-Aeberli, I. The Potential of Prebiotic and Probiotic Supplementation During Obese Pregnancy to Improve Maternal and Offspring’s Metabolic Health and Reduce Obesity Risk—A Narrative Review. Front. Nutr. 2022, 9, 819882. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-Van-meerbeke, A.; Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: A scoping review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Balan, S.S.; Abidin, A.Z.; Perumal, K.V.; Lotafi, A.H.A.; Danabala, S.; Manimaran, M.; Shafie, N.H.; Abdullah, M.A.; Jasni, A.S.; Bahari, H. Effect of elateriospermum tapos extract as coadjuvant in ameliorating maternal obesity on female offspring at weaning. Malaysian J. Microsc. 2019, 15, 111–128. [Google Scholar]
- Abidin, A.Z.; Rosli, N.S.; Segaran, S.; Jasni, A.S.; Bahari, H. Protective effect of Elateriospermum tapos in maternal obesity-induced deficit cognitive function of the offspring. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Nor-Liyana, J.; Siroshini, K.T.; Nurul-Syahirah, M.B.; Chang, W.L.; Nurul-Husna, S.; Daryl, J.A.; Khairul-Kamilah, A.K.; Hasnah, B. Phytochemical analysis of Elateriospermum tapos and its inhibitory effects on alpha-amylase, alpha-glucosidase and pancreatic lipase. J. Trop. For. Sci. 2019, 31, 240–248. [Google Scholar] [CrossRef]
- Yakaiah, V.; Dakshinamoorthi, A.; Sudha Ty, S. Novel Aspects in Inhibiting Pancreatic Lipase with Potential New Compound from Nutmeg in Connection with Obesity-In Vitro, In Silico, In Vivo and Ex Vivo Studies. Maedica-J. Clin. Med. 2021, 16, 445–452. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Leonhardt, W.; Hanefeld, M.; Fischer, S.; Schulze, J. Efficacy of alpha-glucosidase inhibitors on lipids in NIDDM subjects with moderate hyperlipidaemia. Eur. J. Clin. Invest. 1994, 24, 45–49. [Google Scholar] [CrossRef]
- Pattamadilok, D.; Suttisri, R. Seco-Terpenoids and Other Constituents from Elateriospermum tapos. J. Nat. Prod. 2008, 71, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Effect of Kaempferol and Its Glycoside Derivatives on Antioxidant Status of HL-60 Cells Treated with Etoposide. Molecules 2022, 27, 333. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Taheri Zadeh, Z.; Esmaeilpour, K.; Aminzadeh, A.; Heidari, M.R.; Joushi, S. Resveratrol Attenuates Learning, Memory, and Social Interaction Impairments in Rats Exposed to Arsenic. Biomed Res. Int. 2021, 2021, 9993873. [Google Scholar] [CrossRef]
- Koza, L.; Linseman, D. Glutathione precursors shield the brain from trauma. Neural Regen. Res. 2019, 14, 1701. [Google Scholar] [CrossRef] [PubMed]
- Molz, P.; Schröder, N. Potential therapeutic effects of lipoic acid on memory deficits related to aging and neurodegeneration. Front. Pharmacol. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Group/ Fat Tissue | BAT | RpWAT | Visceral | Gonadal |
|---|---|---|---|---|
| NS | 0.11 ± 0.27 a | 0.23 ± 0.06 a | 0.48 ± 0.09 a | 0.49 ± 0.14 a |
| HS | 0.23 ± 0.02 b | 1.08 ± 0.07 b | 1.26 ± 0.05 b | 0.95 ± 0.13 b |
| HY | 0.22 ± 0.01 b | 1.04 ± 0.10 c | 0.88 ± 0.08 c | 0.78 ± 0.02 c |
| HYT5 | 0.13 ± 0.18 a | 0.66 ± 0.09 a | 0.71 ± 0.03 ac | 0.75 ± 0.02 ac |
| HYT50 | 0.12 ± 0.02 a | 0.55 ± 0.04 ac | 0.62 ± 0.06 ac | 0.61 ± 0.05 ac |
| HYT500 | 0.14 ± 0.02 a | 0.61 ± 0.09 a | 0.59 ± 0.07 ac | 0.60 ± 0.03 ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naomi, R.; Rusli, R.N.M.; Huat, T.S.; Embong, H.; Bahari, H.; Kamaruzzaman, M.A. Early Intervention of Elateriospermum tapos Yoghurt in Obese Dams Mitigates Intergenerational Cognitive Deficits and Thigmotactic Behaviour in Male Offspring via the Modulation of Metabolic Profile. Nutrients 2023, 15, 1523. https://doi.org/10.3390/nu15061523
Naomi R, Rusli RNM, Huat TS, Embong H, Bahari H, Kamaruzzaman MA. Early Intervention of Elateriospermum tapos Yoghurt in Obese Dams Mitigates Intergenerational Cognitive Deficits and Thigmotactic Behaviour in Male Offspring via the Modulation of Metabolic Profile. Nutrients. 2023; 15(6):1523. https://doi.org/10.3390/nu15061523
Chicago/Turabian StyleNaomi, Ruth, Rusydatul Nabila Mahmad Rusli, Teoh Soo Huat, Hashim Embong, Hasnah Bahari, and Mohd Amir Kamaruzzaman. 2023. "Early Intervention of Elateriospermum tapos Yoghurt in Obese Dams Mitigates Intergenerational Cognitive Deficits and Thigmotactic Behaviour in Male Offspring via the Modulation of Metabolic Profile" Nutrients 15, no. 6: 1523. https://doi.org/10.3390/nu15061523
APA StyleNaomi, R., Rusli, R. N. M., Huat, T. S., Embong, H., Bahari, H., & Kamaruzzaman, M. A. (2023). Early Intervention of Elateriospermum tapos Yoghurt in Obese Dams Mitigates Intergenerational Cognitive Deficits and Thigmotactic Behaviour in Male Offspring via the Modulation of Metabolic Profile. Nutrients, 15(6), 1523. https://doi.org/10.3390/nu15061523