Iron and DHA in Infant Formula Purchased in the US Fails to Meet European Nutrition Requirements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Formula Categorization
2.3. Regulatory Guidelines
- The Food and Drug Administration (FDA) sets nutrient requirements for infant formula (infants 0–12 months) produced and marketed in the US [4].
- The European Commission sets nutrient requirements for infant formula (infants 0–6 months) and follow-on formula (infants 6–12 months) produced and marketed in the European Union [5].
2.4. Statistical Analysis
3. Results
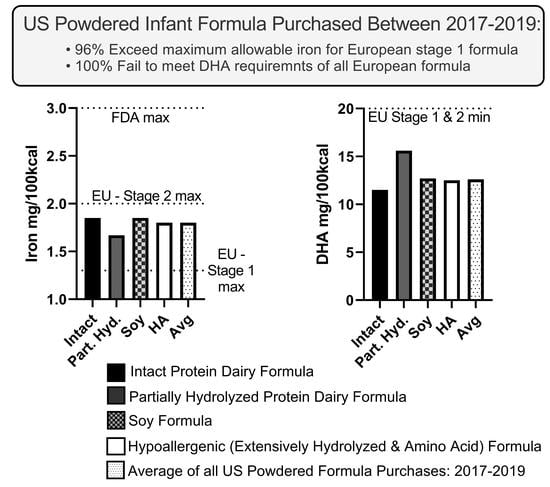
3.1. Iron Content of Formula Purchased
3.2. DHA Content of Formula Purchased
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Exclusive Breastfeeding for Six Months Best for Babies Everywhere; WHO: Geneva, Switzerland, 2011; p. 1. [Google Scholar]
- American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841.
- Center for Disease Control and Prevention (CDC). Breastfeeding Report Card-United States. 2021. Available online: https://www.cdc.gov/breastfeeding/data/reportcard.htm (accessed on 10 January 2023).
- US Food and Drug Administration (FDA). Code of Federal Regulations. Title 21, Volume 2. 21CFR107: Infant Formula; FDA: Washington, DC, USA, 1985. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=107&showFR=1 (accessed on 6 February 2023).
- European Commission. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-on Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding; Official Journal of the European Union: 2016; Volume 59(L25/1). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.025.01.0001.01.ENG (accessed on 6 February 2023).
- CXS 72-1981; Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. CODEX ALIMENTUS: Rome, Italy, 2007.
- CXS 156-1987; Standard for Follow-Up Formula. CODEX ALIMENTUS: Rome, Italy, 2017.
- DiMaggio, D.M.; Du, N.; Porto, A.F. Nutritional and Safety Concerns of Infant Feeding Trends. J. Craniofacial Surg. 2022, 74, 668–673. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). Infant Formula Enforcement Discretion Policy: Guidance for Industry; Office of Nutrition and Food Labeling Center for Food Safety and Applied Nutrition: Rome, Italy, 2022. [Google Scholar]
- US Food and Drug Administration (FDA). Enforcement Discretion to Manufacturers to Increase Infant Formula Supplies-Companies That Received a Letter of Enforcement Discretion for Regular Infant Formula; FDA: Washington, DC, USA, 2023. [Google Scholar]
- Abrams, S.A.; Duggan, C.P. Infant and child formula shortages: Now is the time to prevent recurrences. Am. J. Clin. Nutr. 2022, 116, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Strzalkowski, A.J.; Järvinen, K.M.; Schmidt, B.; Young, B.E. Protein and carbohydrate content of infant formula purchased in the United States. Clin. Exp. Allergy 2022, 52, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Lawrence, R.M. Breastfeeding-A Guide for the Medical Professional, 9th ed.; Elsevier Mosby: Maryland Heights, MO, USA, 2021. [Google Scholar]
- Bosscher, D.; Van Caillie-Bertrand, M.; Robberecht, H.; Van Dyck, K.; Van Cauwenbergh, R.; Deelstra, H. In Vitro Availability of Calcium, Iron, and Zinc from First-Age Infant Formulae and Human Milk. J. Craniofacial Surg. 2001, 32, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friel, J.K.; Andrews, W.L.; Aziz, K.; Kwa, P.G.; Lepage, G.; L’Abbe, M.R. A randomized trial of two levels of iron supplementation and developmental outcome in low birth weight infants. J. Pediatr. 2001, 139, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; De Andraca, I.; Castillo, M.; Smith, J.; Walter, T.; Pino, P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003, 112, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Pino, P.; Pizarro, F.; Lozoff, B. Prevention of iron-deficiency anemia: Comparison of high- and low-iron formulas in term healthy infants after six months of life. J. Pediatr. 1998, 132, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Gahagan, S.; Delker, E.; Blanco, E.; Burrows, R.; Lozoff, B. Randomized Controlled Trial of Iron-Fortified versus Low-Iron Infant Formula: Developmental Outcomes at 16 Years. J. Pediatr. 2019, 212, 124–130.e1. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; A Diersen-Schade, D.; A Boettcher, J.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Périnat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 2017, CD000376. [Google Scholar] [CrossRef] [PubMed]
- Qawasmi, A.; Landeros-Weisenberger, A.; Leckman, J.F.; Bloch, M.H. Meta-analysis of Long-Chain Polyunsaturated Fatty Acid Supplementation of Formula and Infant Cognition. Pediatrics 2012, 129, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drover, J.R.; Felius, J.; Hoffman, D.R.; Castañeda, Y.S.; Garfield, S.; Wheaton, D.H.; Birch, E.E. A randomized trial of DHA intake during infancy: School readiness and receptive vocabulary at 2–3.5 years of age. Early Hum. Dev. 2012, 88, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Carlson, S.E.; Cheatham, C.L.; Shaddy, D.J.; Kerling, E.H.; Thodosoff, J.M.; Gustafson, K.M.; Brez, C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 2013, 98, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Bergmann, K.; Brenna, J.T.; Calder, P.C.; Campoy, C.; Clandinin, M.T.; Colombo, J.; Daly, M.; Decsi, T.; Demmelmair, H.; et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 2020, 111, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Cheung K, P.L.; Helfer, B.; Porubayeva, E.; Dolgikh, E.; Ali, S.; Ali, I.; Archibald-Durham, L.; Brockway, M.; Bugaeva, P.; Chooniedass, R.; et al. International Survey of Health and Nutrition Claims for Infant Formula. Br. Med. J. (BMJ) 2023, in press.



| Regulatory Body | Age (Months) | Formula Type | Minimum | Maximum |
|---|---|---|---|---|
| Iron (mg/100 kcal) | ||||
| Food and Drug Administration | 0–12 | All | 0.15 1 | 3.0 |
| European Commission | 0–6 | Non-Soy-Based | 0.3 | 1.3 |
| 6–12 | Non-Soy-Based | 0.6 | 2.0 | |
| 0–6 | Soy-Based | 0.45 | 2.0 | |
| 6–12 | Soy-Based | 0.9 | 2.5 | |
| CODEX Alimentarius | 0–6 | All | 0.45 | - |
| 6–12 | All | 1.0 | 2.0 | |
| Docosahexaenoic Acid (DHA) (mg/100 kcal) | ||||
| Food and Drug Administration 2 | All | All | Not Required | |
| European Commission 3 | 0–6 | All | 20 | 50 |
| 6–12 | All | 20 | 50 | |
| CODEX Alimentarius 4 | 0–6 | All | Not Required | |
| 6–12 | All | Not Required | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzalkowski, A.; Black, G.; Young, B.E. Iron and DHA in Infant Formula Purchased in the US Fails to Meet European Nutrition Requirements. Nutrients 2023, 15, 1812. https://doi.org/10.3390/nu15081812
Strzalkowski A, Black G, Young BE. Iron and DHA in Infant Formula Purchased in the US Fails to Meet European Nutrition Requirements. Nutrients. 2023; 15(8):1812. https://doi.org/10.3390/nu15081812
Chicago/Turabian StyleStrzalkowski, Alexander, Grace Black, and Bridget E. Young. 2023. "Iron and DHA in Infant Formula Purchased in the US Fails to Meet European Nutrition Requirements" Nutrients 15, no. 8: 1812. https://doi.org/10.3390/nu15081812
APA StyleStrzalkowski, A., Black, G., & Young, B. E. (2023). Iron and DHA in Infant Formula Purchased in the US Fails to Meet European Nutrition Requirements. Nutrients, 15(8), 1812. https://doi.org/10.3390/nu15081812