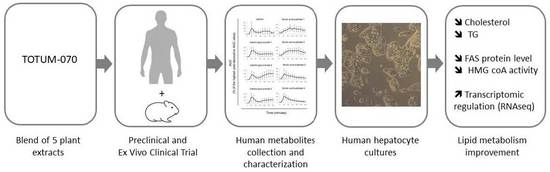
Circulating Human Metabolites Resulting from TOTUM-070 Absorption (a Plant-Based, Polyphenol-Rich Ingredient) Improve Lipid Metabolism in Human Hepatocytes: Lessons from an Original Ex Vivo Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Product
2.2. Ethics Preclincal Study
2.3. Ethics Clinical Trial
2.4. Preclincal Protocol
2.5. Lipid Parameters in Preclinical Study
2.6. Human Study Design and Pharmacokinetic of Absorption
2.7. Phenolic Compounds Extraction from Serum
2.8. Ultra-High Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS/MS)
2.9. Human Hepatocyte Cultures
2.10. Preparation of Palmitate Solution
2.11. Cell Viability
2.12. Red Oil Staining
2.13. Triglycerides Levels
2.14. Cholesterol Levels in HepG2 Cells
2.15. RNA Sequencing
2.16. Fatty Acid Synthase Protein Expression (ELISA)
2.17. HMG-CoA Reductase Activity
2.18. Cell Lysis
2.19. Protein Quantification
2.20. Statistics
3. Results
3.1. Effect of Totum-070 Supplementation on Serum Lipids in Hamsters
3.2. Kinetic Profile of TOTUM-070 Absorption in Humans
3.3. Validation of the Ex Vivo Procedures
3.4. Human Metabolites Deriving from TOTUM-070 Ingestion Limit Palmitate-Induced Intracellular Fat Deposit in Human Hepatocytes
3.5. Human Metabolites from TOTUM-070 Limits Triglycerides and Fully Abolish Palmitate-Related Hepatocytes Cholesterol Content
3.6. TOTUM-070 Human Metabolites Greatly Impact the Expression of Genes Involded in Lipid Metabolism in Human Hepatocytes
3.7. TOTUM-070 Derived Metabolites Limit Lipid Synthesis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Blaha, M.J.; Blankstein, R.; Agatston, A.; Rivera, J.J.; Virani, S.S.; Ouyang, P.; Jones, S.R.; Blumenthal, R.S.; Budoff, M.J.; et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: Implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 2014, 129, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aikawa, M.; Libby, P. The vulnerable atherosclerotic plaque: Pathogenesis and therapeutic approach. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2004, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Barquera, S.; Pedroza-Tobias, A.; Medina, C.; Hernandez-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 1—Full report. J. Clin. Lipidol. 2015, 9, 129–169. [Google Scholar] [CrossRef] [Green Version]
- Ballantyne, C.M.; Banach, M.; Mancini, G.B.J.; Lepor, N.E.; Hanselman, J.C.; Zhao, X.; Leiter, L.A. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: A randomized, placebo-controlled study. Atherosclerosis 2018, 277, 195–203. [Google Scholar] [CrossRef]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- Banach, M.; Stulc, T.; Dent, R.; Toth, P.P. Statin non-adherence and residual cardiovascular risk: There is need for substantial improvement. Int. J. Cardiol. 2016, 225, 184–196. [Google Scholar] [CrossRef]
- Blom, W.A.M.; Koppenol, W.P.; Hiemstra, H.; Stojakovic, T.; Scharnagl, H.; Trautwein, E.A. A low-fat spread with added plant sterols and fish omega-3 fatty acids lowers serum triglyceride and LDL-cholesterol concentrations in individuals with modest hypercholesterolaemia and hypertriglyceridaemia. Eur. J. Nutr. 2019, 58, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Peroni, G.; Riva, A.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Naso, M.; Faliva, M.A.; Tartara, A.; et al. Bergamot phytosome improved visceral fat and plasma lipid profiles in overweight and obese class I subject with mild hypercholesterolemia: A randomized placebo controlled trial. Phytother. Res. PTR 2021, 35, 2045–2056. [Google Scholar] [CrossRef]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinnijenhuis, A.J.; van Holthoon, F.L.; Maathuis, A.J.H.; Vanhoecke, B.; Prawitt, J.; Wauquier, F.; Wittrant, Y. Non-targeted and targeted analysis of collagen hydrolysates during the course of digestion and absorption. Anal. Bioanal. Chem. 2020, 412, 973–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wauquier, F.; Boutin-Wittrant, L.; Pourtau, L.; Gaudout, D.; Moras, B.; Vignault, A.; Monchaux De Oliveira, C.; Gabaston, J.; Vaysse, C.; Bertrand, K.; et al. Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr’Inside(TM)) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders. Nutrients 2022, 14, 1511. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef]
- Wauquier, F.; Daneault, A.; Granel, H.; Prawitt, J.; Fabien Soule, V.; Berger, J.; Pereira, B.; Guicheux, J.; Rochefort, G.Y.; Meunier, N.; et al. Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: From Bedside to Bench and Vice Versa. Nutrients 2019, 11, 1249. [Google Scholar] [CrossRef] [Green Version]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [Google Scholar] [CrossRef] [Green Version]
- Nikasa, P.; Tricot, T.; Mahdieh, N.; Baharvand, H.; Totonchi, M.; Hejazi, M.S.; Verfaillie, C.M. Patient-Specific Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cells as a Model to Study Autosomal Recessive Hypercholesterolemia. Stem Cells Dev. 2021, 30, 714–724. [Google Scholar] [CrossRef]
- Yu, H.; Rimbert, A.; Palmer, A.E.; Toyohara, T.; Xia, Y.; Xia, F.; Ferreira, L.M.R.; Chen, Z.; Chen, T.; Loaiza, N.; et al. GPR146 Deficiency Protects against Hypercholesterolemia and Atherosclerosis. Cell 2019, 179, 1276–1288.e14. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; He, L.; Pu, W.; Yu, W.; Li, Y.; Wu, Y.T.; Xu, C.; Wei, Y.; Ding, Q.; et al. In Vivo AAV-CRISPR/Cas9-Mediated Gene Editing Ameliorates Atherosclerosis in Familial Hypercholesterolemia. Circulation 2020, 141, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielcka, E.; Horoszkiewicz-Hassan, M. The influence of supplementation with artichoke (Cynara scolymus L.) extract on selected redox parameters in rowers. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Salem, M.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological Studies of Artichoke Leaf Extract and Their Health Benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Wallis, C.; Simpson, H.C. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomed. Int. J. Phytother. Phytopharm. 2008, 15, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Englisch, W.; Beckers, C.; Unkauf, M.; Ruepp, M.; Zinserling, V. Efficacy of Artichoke dry extract in patients with hyperlipoproteinemia. Arzneim.-Forsch. 2000, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Faliva, M.A.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef]
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2013, 3, CD003335. [Google Scholar] [CrossRef]
- Ben Salem, M.; Ksouda, K.; Dhouibi, R.; Charfi, S.; Turki, M.; Hammami, S.; Ayedi, F.; Sahnoun, Z.; Zeghal, K.M.; Affes, H. LC-MS/MS Analysis and Hepatoprotective Activity of Artichoke (Cynara scolymus L.) Leaves Extract against High Fat Diet-Induced Obesity in Rats. BioMed Res. Int. 2019, 2019, 4851279. [Google Scholar] [CrossRef] [Green Version]
- Kwon, E.Y.; Kim, S.Y.; Choi, M.S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients 2018, 10, 979. [Google Scholar] [CrossRef] [Green Version]
- Honore-Thorez, D. Description, identification and therapeutic use of Chrysanthellum “americanum”: Chrysanthellum indicum DC. subsp afroamericanum B. L. Turner. J. de Pharm. de Belg. 1985, 40, 323–331. [Google Scholar]
- Guo, X.F.; Li, Z.H.; Cai, H.; Li, D. The effects of Lycium barbarum L. (L. barbarum) on cardiometabolic risk factors: A meta-analysis of randomized controlled trials. Food Funct. 2017, 8, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Chernukha, I.M.; Kotenkova, E.A.; Vasilevskaya, E.R.; Ivankin, A.N.; Lisitsyn, A.B.; Fedulova, L.V. The study of biological effects of different geographical origin goji berries in rats with alimentary hypercholesterolemia. Vopr. Pitan. 2020, 89, 37–45. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Olmez, E.; Vural, K.; Gok, S.; Ozturk, Z.; Kayalar, H.; Ayhan, S.; Var, A. Olive Leaf Extract Improves the Atherogenic Lipid Profile in Rats Fed a High Cholesterol Diet. Phytother. Res. 2015, 29, 1652–1657. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Lu, H.; Guo, F.; Han, C.; Sun, G. Practical Application of Antidiabetic Efficacy of Lycium barbarum Polysaccharide in Patients with Type 2 Diabetes. Med. Chem. 2015, 11, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef] [Green Version]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Huber, R.; Muller, M.; Naumann, J.; Schenk, T.; Ludtke, R. Artichoke leave extract for chronic hepatitis C—A pilot study. Phytomed. Int. J. Phytother. Phytopharm. 2009, 16, 801–804. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99 (Suppl. S3), S55–S58. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Petrangolini, G.; Allegrini, P.; Perna, S.; Giacosa, A.; Peroni, G.; Faliva, M.A.; Naso, M.; Rondanelli, M. Artichoke and Bergamot Phytosome Alliance: A Randomized Double Blind Clinical Trial in Mild Hypercholesterolemia. Nutrients 2021, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Capurso, C.; Capurso, A.; Massaro, M. Vascular effects of the Mediterranean diet-part II: Role of omega-3 fatty acids and olive oil polyphenols. Vasc. Pharmacol. 2014, 63, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Atal, N.; Bedi, K.L. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabater, C.; Blanco-Doval, A.; Margolles, A.; Corzo, N.; Montilla, A. Artichoke pectic oligosaccharide characterisation and virtual screening of prebiotic properties using in silico colonic fermentation. Carbohydr. Polym. 2021, 255, 117367. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Villar, A.; Zangara, A.; Smidt, C.R.; Risco, E. In Vitro Evaluation of Prebiotic Properties of a Commercial Artichoke Inflorescence Extract Revealed Bifidogenic Effects. Nutrients 2020, 12, 1552. [Google Scholar] [CrossRef]
- Sun, Q.; Du, M.; Kang, Y.; Zhu, M.J. Prebiotic effects of goji berry in protection against inflammatory bowel disease. Crit. Rev. Food Sci. Nutr. 2022, 6, 1–25. [Google Scholar] [CrossRef]
- Schumacher, M.M.; DeBose-Boyd, R.A. Posttranslational Regulation of HMG CoA Reductase, the Rate-Limiting Enzyme in Synthesis of Cholesterol. Annu. Rev. Biochem. 2021, 90, 659–679. [Google Scholar] [CrossRef]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain, C.M.N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2019, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Bashore, A.C.; Liu, M.; Key, C.C.; Boudyguina, E.; Wang, X.; Carroll, C.M.; Sawyer, J.K.; Mullick, A.E.; Lee, R.G.; Macauley, S.L.; et al. Targeted Deletion of Hepatocyte Abca1 Increases Plasma HDL (High-Density Lipoprotein) Reverse Cholesterol Transport via the LDL (Low-Density Lipoprotein) Receptor. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1747–1761. [Google Scholar] [CrossRef] [PubMed]










| geneSet | Description | Overlap | enrichmentRatio | p-Value |
|---|---|---|---|---|
| hsa03010 | Ribosome | 87 | 3.8442376877825852 | 0 |
| hsa03050 | Proteasome | 30 | 3.947339847991314 | 1.2612133559741778 × 1013 |
| hsa00100 | Steroid biosynthesis | 10 | 3.1163209326247214 | 3.852518584954723 × 104 |
| hsa00220 | Arginine biosynthesis | 10 | 2.8195284628509385 | 0.0010621371529087043 |
| hsa01040 | Biosynthesis of unsaturated fatty acids | 12 | 2.6315598986608757 | 7.355634194528005 × 104 |
| hsa01212 | Fatty acid metabolism | 21 | 2.5904417752442996 | 1.1116706622127381 × 105 |
| hsa00062 | Fatty acid elongation | 12 | 2.3684039087947886 | 0.002233923233486923 |
| hsa04115 | p53 signaling pathway | 28 | 2.3026149113282663 | 6.596143085291217 × 106 |
| hsa04979 | Cholesterol metabolism | 18 | 2.131563517915309 | 8.662155586620646 × 104 |
| hsa00190 | Oxidative phosphorylation | 47 | 2.0923869119051703 | 1.684037256310944 × 107 |
| hsa03320 | PPAR signaling pathway | 26 | 2.080354784752179 | 1.0736921306331304 × 104 |
| hsa04714 | Thermogenesis | 78 | 2.0167631537772217 | 1.070485922127773 × 1010 |
| hsa03040 | Spliceosome | 45 | 2.0033491709730353 | 1.2521413876864784 × 106 |
| hsa05012 | Parkinson disease | 47 | 1.9597708400238565 | 1.4871034260677263 × 106 |
| hsa05130 | Pathogenic Escherichia coli infection | 18 | 1.937785016286645 | 0.0029800691866965767 |
| hsa05016 | Huntington disease | 62 | 1.9020860407419287 | 1.0991630317036538 × 107 |
| hsa04932 | Non-alcoholic fatty liver disease (NAFLD) | 47 | 1.8677010690160243 | 6.65595639137706 × 106 |
| hsa00240 | Pyrimidine metabolism | 31 | 1.8173396329860998 | 4.129936339646312 × 104 |
| hsa04211 | Longevity regulating pathway | 27 | 1.7962613915016652 | 0.0011583288792791357 |
| hsa03008 | Ribosome biogenesis in eukaryotes | 24 | 1.7543732657739173 | 0.0030116440823730883 |
| hsa05222 | Small cell lung cancer | 27 | 1.737687650474437 | 0.0019929850989139908 |
| hsa05010 | Alzheimer disease | 49 | 1.6966636188734594 | 7.322855774782866 × 105 |
| hsa04152 | AMPK signaling pathway | 33 | 1.628277687296417 | 0.0022440329320105867 |
| hsa04218 | Cellular senescence | 41 | 1.5172587540716613 | 0.0030390963377235902 |
| hsa05169 | Epstein-Barr virus infection | 50 | 1.4728880029818336 | 0.002243922870621695 |
| hsa01100 | Metabolic pathways | 261 | 1.184201954397394 | 6.418137152468528 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wauquier, F.; Boutin-Wittrant, L.; Krisa, S.; Valls, J.; Langhi, C.; Otero, Y.F.; Sirvent, P.; Peltier, S.; Bargetto, M.; Cazaubiel, M.; et al. Circulating Human Metabolites Resulting from TOTUM-070 Absorption (a Plant-Based, Polyphenol-Rich Ingredient) Improve Lipid Metabolism in Human Hepatocytes: Lessons from an Original Ex Vivo Clinical Trial. Nutrients 2023, 15, 1903. https://doi.org/10.3390/nu15081903
Wauquier F, Boutin-Wittrant L, Krisa S, Valls J, Langhi C, Otero YF, Sirvent P, Peltier S, Bargetto M, Cazaubiel M, et al. Circulating Human Metabolites Resulting from TOTUM-070 Absorption (a Plant-Based, Polyphenol-Rich Ingredient) Improve Lipid Metabolism in Human Hepatocytes: Lessons from an Original Ex Vivo Clinical Trial. Nutrients. 2023; 15(8):1903. https://doi.org/10.3390/nu15081903
Chicago/Turabian StyleWauquier, Fabien, Line Boutin-Wittrant, Stéphanie Krisa, Josep Valls, Cedric Langhi, Yolanda F. Otero, Pascal Sirvent, Sébastien Peltier, Maxime Bargetto, Murielle Cazaubiel, and et al. 2023. "Circulating Human Metabolites Resulting from TOTUM-070 Absorption (a Plant-Based, Polyphenol-Rich Ingredient) Improve Lipid Metabolism in Human Hepatocytes: Lessons from an Original Ex Vivo Clinical Trial" Nutrients 15, no. 8: 1903. https://doi.org/10.3390/nu15081903
APA StyleWauquier, F., Boutin-Wittrant, L., Krisa, S., Valls, J., Langhi, C., Otero, Y. F., Sirvent, P., Peltier, S., Bargetto, M., Cazaubiel, M., Sapone, V., Bouchard-Mercier, A., Roux, V., Macian, N., Pickering, G., & Wittrant, Y. (2023). Circulating Human Metabolites Resulting from TOTUM-070 Absorption (a Plant-Based, Polyphenol-Rich Ingredient) Improve Lipid Metabolism in Human Hepatocytes: Lessons from an Original Ex Vivo Clinical Trial. Nutrients, 15(8), 1903. https://doi.org/10.3390/nu15081903