Associations between Maternal Nutritional Status, Hemodynamic Parameters, and Delivery Outcomes in Low-Risk Pregnancies: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Recruitment
2.2. Statistical Analysis
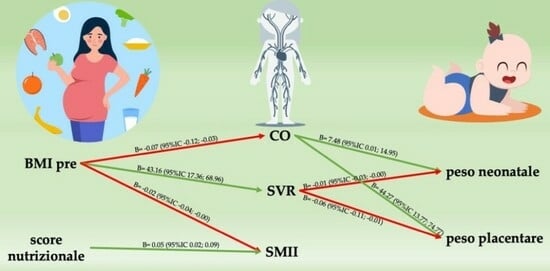
3. Results
4. Discussion
4.1. Main Findigs
4.2. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| USCOM | Ultrasonic Cardiac Output Monitor |
| CO | cardiac output |
| SVR | systemic vascular resistance |
| SMII | Smith–Madigan inotropy index |
| BMI | body mass index |
| HR | heart rate |
| SV | stroke volume |
| MAP | mean arterial pressure |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| FGR | fetal growth restriction |
| pPROM | preterm premature rupture of the membranes |
| GDM | gestational diabetes |
| CI | cardiac output index |
| SVRI | systemic vascular resistance index |
| TNF-a | Tumor necrosis factor-alpha |
| GWG | gestational weight gain |
| FIGO | Federation of Gynecology and Obstetrics |
| BSA | body surface area |
| FPR | fetal/placental weight ratio |
| ART | assisted reproductive technology |
| BE | base excess |
| LDL | low-density lipoproteins |
| PlGF | Placental Growth Factor |
References
- Curtis, S.L.; Belham, M.; Bennett, S.; James, R.; Harkness, A.; Gamlin, W.; Thilaganathan, B.; Giorgione, V.; Douglas, H.; Carroll, A.; et al. Transthoracic Echocardiographic Assessment of the Heart in Pregnancy—A position statement on behalf of the British Society of Echocardiography and the United Kingdom Maternal Cardiology Society. Echo Res. Pract. 2023, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Ramlakhan, K.P.; Johnson, M.R.; Roos-Hesselink, J.W. Pregnancy and cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Farsetti, D.; Pisani, I.; Tiralongo, G.M.; Presti, D.L.; Gagliardi, G.; Vasapollo, B.; Novelli, G.P. Friendly help for clinical use of maternal hemodynamics. J. Matern. Fetal Neonatal Med. 2021, 34, 3075–3079. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, D.; Patey, O.; Thilaganathan, B.; Khalil, A. Cardiac output assessment in pregnancy: Comparison of two automated monitors with echocardiography. Ultrasound Obstet. Gynecol. 2017, 49, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kager, C.C.M.; Dekker, G.A.; Stam, M.C. Measurement of cardiac output in normal pregnancy by a non-invasive two-dimensional independent doppler device. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.W.; Peyton, P.J. A meta-analysis of the accuracy and precision of the ultrasonic cardiac output monitor (USCOM). Anaesthesia 2012, 67, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Lichtenberg, A.; Winterhalter, M.; Rossner, D.; Pichlmaier, M.; Phillips, R. Non-invasive cardiac output determination by two-dimensional independent Doppler during and after cardiac surgery. Ann. Thorac. Surg. 2005, 80, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.; Schneider, K.A.; Zang, H.; Batlivala, S.P.; Monteleone, M.P.; Benscoter, A.L.; Chlebowski, M.M.; Iliopoulos, I.D.; Cooper, D.S. Measurement of Cardiac Output Using an Ultrasonic Cardiac Output Monitor (USCOM) in Patients with Single-Ventricle Physiology. Pediatr. Cardiol. 2022, 43, 1205–1213. [Google Scholar] [CrossRef]
- Mulder, E.; Basit, S.; Oben, J.; van Kuijk, S.; Ghossein-Doha, C.; Spaanderman, M. Accuracy and precision of USCOM versus transthoracic echocardiography before and during pregnancy. Pregnancy Hypertens 2019, 17, 138–143. [Google Scholar] [CrossRef]
- Vinayagam, D.; Thilaganathan, B.; Stirrup, O.; Mantovani, E.; Khalil, A. Maternal hemodynamics in normal pregnancy: Reference ranges and role of maternal characteristics. Ultrasound Obstet. Gynecol. 2018, 51, 665–671. [Google Scholar] [CrossRef]
- Montaguti, E.; Youssef, A.; Cavalera, M.; Dodaro, M.G.; Cofano, M.; Fiorentini, M.; Pellegrino, A.; Pilu, G. Maternal hemodynamic assessment by USCOM® device in the first trimester of pregnancy. J. Matern. Fetal Neonatal Med. 2022, 35, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Farsetti, D.; Vasapollo, B.; Pometti, F.; Frantellizzi, R.; Novelli, G.P.; Valensise, H. Maternal hemodynamics for the identification of early fetal growth restriction in normotensive pregnancies. Placenta 2022, 129, 12–14. [Google Scholar] [CrossRef]
- Ornaghi, S.; Caricati, A.; Di Martino, D.D.; Mossa, M.; Di Nicola, S.; Invernizzi, F.; Zullino, S.; Clemenza, S.; Barbati, V.; Tinè, G.; et al. Non-invasive Maternal Hemodynamic Assessment to Classify High-Risk Pregnancies Complicated by Fetal Growth Restriction. Front. Clin. Diabetes Healthc. 2022, 3, 851971. [Google Scholar] [CrossRef] [PubMed]
- Farsetti, D.; Pometti, F.; Tiralongo, G.M.; Presti, D.L.; Pisani, I.; Gagliardi, G.; Vasapollo, B.; Novelli, G.P.; Valensise, H. Distinction between SGA and FGR by means of fetal umbilical vein flow and maternal hemodynamics. J. Matern. Fetal Neonatal Med. 2022, 35, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall’asta, A.; DeVore, G.R.; Prefumo, F.; Frusca, T.; Visser, G.H.; Hobbins, J.C.; Baschat, A.A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Montaguti, E.; Cofano, M.; Diglio, J.; Fiorentini, M.; Pellegrino, A.; Lenzi, J.; Battaglia, C.; Pilu, G. The prediction of hypertensive disorders by maternal hemodynamic assessment in the first trimester of pregnancy. J. Matern. Fetal Neonatal Med. 2023, 36, 2198063. [Google Scholar] [CrossRef]
- Perry, H.; Binder, J.; Gutierrez, J.; Thilaganathan, B.; Khalil, A. Maternal haemodynamic function differs in pre-eclampsia when it is associated with a small-for-gestational-age newborn: A prospective cohort study. BJOG 2021, 128, 167–175. [Google Scholar] [CrossRef]
- Valensise, H.; Pometti, F.; Farsetti, D.; Novelli, G.P.; Vasapollo, B. Hemodynamic assessment in patients with preterm premature rupture of the membranes (pPROM). Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 1–4. [Google Scholar] [CrossRef]
- Mecacci, F.; Ottanelli, S.; Vannuccini, S.; Clemenza, S.; Lisi, F.; Serena, C.; Rambaldi, M.P.; Simeone, S.; Pisani, I.; Petraglia, F.; et al. Maternal hemodynamic changes in gestational diabetes: A prospective case-control study. Arch. Gynecol. Obstet. 2022, 306, 357–363. [Google Scholar] [CrossRef]
- Vinayagam, D.; Gutierrez, J.; Binder, J.; Mantovani, E.; Thilaganathan, B.; Khalil, A. Impaired maternal hemodynamics in morbidly obese women: A case-control study. Ultrasound Obstet. Gynecol. 2017, 50, 761–765. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Zavatta, A.; Parisi, F.; Mandò, C.; Scaccabarozzi, C.; Savasi, V.M.; Cetin, I. Role of Inflammaging on the Reproductive Function and Pregnancy. Clin. Rev. Allergy Immunol. 2022, 64, 145–160. [Google Scholar] [CrossRef] [PubMed]
- St-Germain, L.E.; Castellana, B.; Baltayeva, J.; Beristain, A.G. Maternal Obesity and the Uterine Immune Cell Landscape: The Shaping Role of Inflammation. Int. J. Mol. Sci. 2020, 21, 3776. [Google Scholar] [CrossRef] [PubMed]
- Musa, E.; Salazar-Petres, E.; Arowolo, A.; Levitt, N.; Matjila, M.; Sferruzzi-Perri, A.N. Obesity and gestational diabetes independently and collectively induce specific effects on placental structure, inflammation and endocrine function in a cohort of South African women. J. Physiol. 2023, 601, 1287–1306. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.T.; Swertfeger, D.K.; Morris, J.; Street, S.E.; Warshak, C.R.; Welge, J.A.; Remaley, A.T.; Catov, J.M.; Davidson, W.S.; Woollett, L.A. Pregnancy is accompanied by larger high density lipoprotein particles and compositionally distinct subspecies. J. Lipid Res. 2021, 62, 100107. [Google Scholar] [CrossRef] [PubMed]
- Reichetzeder, C. Overweight and obesity in pregnancy: Their impact on epigenetics. Eur. J. Clin. Nutr. 2021, 75, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Kodogo, V.; Azibani, F.; Sliwa, K. Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: A literature review. Clin. Res. Cardiol. 2019, 108, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Paszkowski, T.; Cetin, I.; Castelo-Branco, C. Supplementation during pregnancy: Beliefs and science. Gynecol. Endocrinol. 2016, 32, 509–516. [Google Scholar] [CrossRef]
- Papageorghiou, A.; Papageorghiou, A.T.; Ohuma, E.O.; Gravett, M.G.; Hirst, J.; da Silveira, M.F.; Lambert, A.; Carvalho, M.; Jaffer, Y.A.; Altman, D.G.; et al. International standards for symphysis-fundal height based on serial measurements from the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: Prospective cohort study in eight countries on behalf of the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). BMJ 2016, 355, i5662. [Google Scholar] [CrossRef]
- Parisi, F.; Savasi, V.M.; Di Bartolo, I.; Mandia, L.; Cetin, I. Associations between First Trimester Maternal Nutritional Score, Early Markers of Placental Function, and Pregnancy Outcome. Nutrients 2020, 12, 799. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Rousian, M.; Steegers-Theunissen, R.P.M.; Koning, A.H.J.; Willemsen, S.P.; de Vries, J.H.M.; Cetin, I.; Steegers, E.A.P. Early first trimester maternal “high fish and olive oil and low meat” dietary pattern is associated with accelerated human embryonic development. Eur. J. Clin. Nutr. 2018, 72, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Anelli, G.M.; Parisi, F.; Sarno, L.; Fornaciari, O.; Carlea, A.; Coco, C.; Della Porta, M.; Mollo, N.; Villa, P.M.; Guida, M.; et al. Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study. Nutrients 2022, 14, 3631. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Milazzo, R.; Savasi, V.M.; Cetin, I. Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. Int. J. Mol. Sci. 2021, 22, 1732. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S4), S213–S253. [Google Scholar] [CrossRef]
- Jacob, C.M.; Inskip, H.M.; Lawrence, W.; McGrath, C.; McAuliffe, F.M.; Killeen, S.L.; Divakar, H.; Hanson, M. Acceptability of the FIGO Nutrition Checklist in Preconception and Early Pregnancy to Assess Nutritional Status and Prevent Excess Gestational Weight Gain: A Study of Women and Healthcare Practitioners in the UK. Nutrients 2022, 14, 3623. [Google Scholar] [CrossRef]
- Teulings, N.E.W.D.; Wood, A.M.; Sovio, U.; Ozanne, S.E.; Smith, G.C.S.; Aiken, C.E. Independent influences of maternal obesity and fetal sex on maternal cardiovascular adaptation to pregnancy: A prospective cohort study. Int. J. Obes. 2020, 44, 2246–2255. [Google Scholar] [CrossRef]
- Manrique-Acevedo, C.; Chinnakotla, B.; Padilla, J.; Martinez-Lemus, L.A.; Gozal, D. Obesity and cardiovascular disease in women. Int. J. Obes. 2020, 44, 1210–1226. [Google Scholar] [CrossRef]
- Vonck, S.; Lanssens, D.; Staelens, A.S.; Tomsin, K.; Oben, J.; Bruckers, L.; Gyselaers, W. Obesity in pregnancy causes a volume overload in third trimester. Eur. J. Clin. Investig. 2019, 49, e13173. [Google Scholar] [CrossRef]
- Spradley, F.T.; Palei, A.C.; Granger, J.P.; Qu, H.; Khalil, R.A.; Beckers, K.F.; Sones, J.L.; Gandhi, K.; Li, C.; German, N.; et al. Increased risk for the development of preeclampsia in obese pregnancies: Weighing in on the mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1326–R1343. [Google Scholar] [CrossRef]
- Anil Kumar, K.V.; Kavitha, S.; Sreekanth, K.S. Regulatory proteins in placental angiogenesis. Biomedicine 2021, 41, 694–700. [Google Scholar] [CrossRef]
- Mohammadifard, N.; Gotay, C.; Humphries, K.H.; Ignaszewski, A.; Esmaillzadeh, A.; Sarrafzadegan, N. Electrolyte minerals intake and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2019, 59, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Mecacci, F.; Avagliano, L.; Lisi, F.; Clemenza, S.; Serena, C.; Vannuccini, S.; Rambaldi, M.P.; Simeone, S.; Ottanelli, S.; Petraglia, F. Fetal Growth Restriction: Does an Integrated Maternal Hemodynamic-Placental Model Fit Better? Reprod. Sci. 2021, 28, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W.; Lees, C. Maternal Low Volume Circulation Relates to Normotensive and Preeclamptic Fetal Growth Restriction. Front. Med. 2022, 9, 902634. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Flores-Mateo, G.; Sánchez, E. Dietary patterns and CVD: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2015, 114, 1341–1359. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Abdolshahi, A.; Shab-Bidar, S. Healthy and unhealthy dietary patterns and the risk of chronic disease: An umbrella review of meta-analyses of prospective cohort studies. Br. J. Nutr. 2020, 124, 1133–1144. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and vascular effect of the mediterranean diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef]
| Maternal Characteristics | Total Study Population (n = 143) | |
|---|---|---|
| Maternal age, years, median (range) | 33 (20–43) | |
| Maternal pregestational BMI, kg/m2, median (range) | 21.0 (16.6–38.5) | |
| Pregestational BMI, kg/m2, n (%) | 18.5–24.9 | 110 (76.9) |
| 25–29.9 | 18 (12.6) | |
| ≥30 | 3 (2.1) | |
| GWG at enrollment, kg, median (range) | 12 (3–24) | |
| Gestational age at enrollment, days, median (range) | 270 (254–286) | |
| Educational level, n (%) | low | 6 (4.2) |
| intermediate | 33 (23.1) | |
| high | 100 (69.9) | |
| Working status, n (%) | unemployed | 19 (13.3) |
| employed | 118 (82.5) | |
| Marital status, n (%) | not married | 62 (43.4) |
| married | 77 (53.8) | |
| Ethnicity, n (%) | non-Caucasian | 19 (13.3) |
| Caucasian | 124 (86.7) | |
| Conception mode, n (%) | spontaneous | 137 (95.8%) |
| ART | 6 (4.2%) | |
| Smoking habit, yes n (%) | yes | 5 (3.5) |
| Nulliparous, n (%) | 95 (66.4) | |
| FIGO nutritional score, median (range) | 7 (3–10) | |
| Score Component | Frequency (n = 143) |
|---|---|
| Meat, yes n (%) | 113 (79.0) |
| Fruit and vegetables, yes n (%) | 113 (79.0) |
| Fish, yes n (%) | 112 (78.3) |
| Dairy, yes n (%) | 113 (79.0) |
| Whole cereals, yes n (%) | 81 (56.6) |
| Sweets and snacks, yes n (%) | 95 (66.4) |
| Hemoglobin, yes n (%) | 112 (78.3) |
| Folic acid, yes n (%) | 133 (93.0) |
| Iodized salt, yes n (%) | 122 (85.3) |
| Sun exposure, yes n (%) | 74 (51.7) |
| Hemodynamic Parameters | Total Study Population (n = 143) |
|---|---|
| BSA, median (range), m2 | 1.8 (1.4–2.3) |
| HR, median (range), bpm | 77.7 (44.7–123.4) |
| SV, median (range), cm3 | 44.0 (25.0–68.8) |
| CO, median (range), L/min | 6.4 (3.4–9.6) |
| MAP, median (range), mmHg | 80 (60–100) |
| SVR, median (range), dyne s cm−5 | 1000.0 (609.4–1651.4) |
| SMII, median (range), Watt/m2 | 1.6 (0.9–2.6) |
| Delivery Outcomes | Total Study Population (n = 139) | |
|---|---|---|
| GWG at delivery, kg, median (range) | 12.0 (4.0–25.0) | |
| Gestational age at delivery, days, median (range) | 281 (267–292) | |
| Delivery mode, n (%) | Vaginal delivery | 105 (75.5) |
| Vacuum-assisted operative delivery | 10 (7.2) | |
| Caesarean section | 24 (17.3) | |
| Birthweight, g, median (range) | 3405 (2500–4630) | |
| Birthweight, centile, median (range) | 51 (4–99) | |
| Blood loss, mL, median (range) | 400 (100–1550) | |
| Placental weight, g, median (range) | 550 (350–890) | |
| FPR, median (range) | 6.1 (4.5–10.1) | |
| pH, median (range) | 7.24 (6.98–7.53) | |
| BE, median (range) | −5.0 (−16.3–4.2) | |
| Lactate, median (range) | 4.0 (1.2–12.9) | |
| Apgar-1, median (range) | 9 (6–10) | |
| Apgar-5, median (range) | 10 (8–10) | |
| Neonatal sex, n (%) | Male | 71 (51) |
| Female | 68 (49) | |
| Independent Variable | Dependent Variable | β (95%CI) | p-Value |
|---|---|---|---|
| BMI | CO | −0.07 (−0.12; −0.03) | <0.001 |
| SV | −0.92 (−1,47; −0.37) | 0.001 | |
| SVR | 43.16 (17.36; 68.96) | <0.001 | |
| SMII | −0.02 (−0.04; −0.00) | 0.02 | |
| Nutritional score | SMII | 0.05 (0.02; 0.09) | 0.005 |
| CO | Neonatal weight | 7.48 (0.01; 14.95) | 0.05 |
| Placental weight | 44.27 (13.77; 74.77) | <0.01 | |
| SVR | Neonatal weight | −0.01 (−0.03; −0.01) | 0.02 |
| Placental weight | −0.06 (−0.11; −0.01) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubrano, C.; Parisi, F.; Coco, C.; Marelli, E.; Burello, E.; Cetin, I. Associations between Maternal Nutritional Status, Hemodynamic Parameters, and Delivery Outcomes in Low-Risk Pregnancies: A Prospective Observational Study. Nutrients 2024, 16, 183. https://doi.org/10.3390/nu16020183
Lubrano C, Parisi F, Coco C, Marelli E, Burello E, Cetin I. Associations between Maternal Nutritional Status, Hemodynamic Parameters, and Delivery Outcomes in Low-Risk Pregnancies: A Prospective Observational Study. Nutrients. 2024; 16(2):183. https://doi.org/10.3390/nu16020183
Chicago/Turabian StyleLubrano, Chiara, Francesca Parisi, Chiara Coco, Elisabetta Marelli, Eleonora Burello, and Irene Cetin. 2024. "Associations between Maternal Nutritional Status, Hemodynamic Parameters, and Delivery Outcomes in Low-Risk Pregnancies: A Prospective Observational Study" Nutrients 16, no. 2: 183. https://doi.org/10.3390/nu16020183
APA StyleLubrano, C., Parisi, F., Coco, C., Marelli, E., Burello, E., & Cetin, I. (2024). Associations between Maternal Nutritional Status, Hemodynamic Parameters, and Delivery Outcomes in Low-Risk Pregnancies: A Prospective Observational Study. Nutrients, 16(2), 183. https://doi.org/10.3390/nu16020183