Effects of Prolonged Medical Fasting during an Inpatient, Multimodal, Nature-Based Treatment on Pain, Physical Function, and Psychometric Parameters in Patients with Fibromyalgia: An Observational Study
Abstract
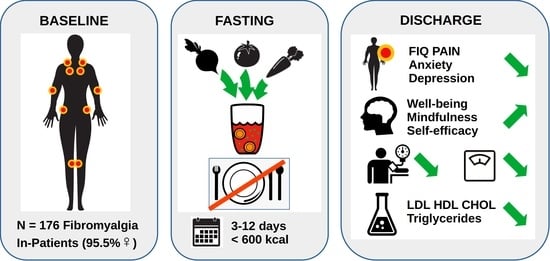
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Interventions
2.4. Participants
2.5. Variables
2.6. Data Sources/Measurement
2.7. Bias
2.8. Study Size
2.9. Statistical Methods
3. Results
3.1. Study Population
3.2. Questionnaires
3.3. Physiological Parameters
3.4. Safety and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional Interventions in the Management of Fibromyalgia Syndrome. Nutrients 2020, 12, 2525. [Google Scholar] [CrossRef] [PubMed]
- Lowry, E.; Marley, J.; McVeigh, J.G.; McSorley, E.; Allsopp, P.; Kerr, D. Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients 2020, 12, 2664. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Berwick, R.; Barker, C.; Goebel, A. The diagnosis of fibromyalgia syndrome. Clin. Med. 2022, 22, 570–574. [Google Scholar]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kaleycheva, N.; Cullen, A.E.; Evans, R.; Harris, T.; Nicholson, T.; Chalder, T. The role of lifetime stressors in adult fibromyalgia: Systematic review and meta-analysis of case-control studies. Psychol. Med. 2021, 51, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Munipalli, B.; Allman, M.E.; Chauhan, M.; Niazi, S.K.; Rivera, F.; Abril, A.; Wang, B.; Wieczorek, M.A.; Hodge, D.O.; Knight, D.; et al. Depression: A Modifiable Risk Factor for Poor Outcomes in Fibromyalgia. J. Prim. Care Community Health 2022, 13, 21501319221120738. [Google Scholar] [CrossRef]
- Habibi Asgarabad, M.; Salehi Yegaei, P.; Jafari, F.; Azami-Aghdash, S.; Lumley, M.A. The relationship of alexithymia to pain and other symptoms in fibromyalgia: A systematic review and meta-analysis. Eur. J. Pain. 2022, 27, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Kundakci, B.; Hall, M.; Atzeni, F.; Branco, J.; Buskila, D.; Clauw, D.; Crofford, L.J.; Fitzcharles, M.A.; Georgopoulos, V.; Gerwin, R.D.; et al. International, multidisciplinary Delphi consensus recommendations on non-pharmacological interventions for fibromyalgia. Semin. Arthritis Rheum. 2022, 57, 152101. [Google Scholar] [CrossRef]
- Rico-Villademoros, F.; Postigo-Martin, P.; Garcia-Leiva, J.M.; Ordoñez-Carrasco, J.L.; Calandre, E.P. Patterns of pharmacologic and non-pharmacologic treatment, treatment satisfaction and perceived tolerability in patients with fibromyalgia: A patients’ survey. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 123), 72–78. [Google Scholar] [PubMed]
- Valladales-Restrepo, L.F.; Oyuela-Gutiérrez, M.C.; Alzate-García, M.; Osorio-Rodas, I.; Quintero-Flórez, V.; Restrepo-Muñoz, J.F.; Suárez-Cardona, J.A.; Barroso-Fernandes, S.T.; Machado-Alba, J.E. Treatment patterns in fibromyalgia including the use of opioids. Musculoskeletal Care 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Bradley, M.J.; Onat, A.M.; Shi, H.N.; Zheng, S. Review of nutritional approaches to fibromyalgia. Nutr. Rev. 2022, 80, 2260–2274. [Google Scholar] [CrossRef]
- Stubbs, A.; Harte, S.; Clauw, D.J.; Williams, D.A.; McAfee, J.; Miller, N.; Brown, M.; Med, C.N.; Rothberg, A.; Schrepf, A. Early Relationships of a Low-Energy Diet With Symptoms of Fibromyalgia. ACR Open Rheumatol. 2022, 4, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Aguilar, E.; Marcos-Pasero, H.; Ikonomopoulou, M.P.; Loria-Kohen, V. Food Implications in Central Sensitization Syndromes. J. Clin. Med. 2020, 9, 4106. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, Y.; Miralles-Amorós, L.; Martínez-Olcina, M.; Sánchez-Ortega, M.; Mora, J.; Martínez-Rodríguez, A. Vegetarian and Vegan Diet in Fibromyalgia: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 4955. [Google Scholar] [CrossRef]
- Baldi, S.; Pagliai, G.; Dinu, M.; Di Gloria, L.; Nannini, G.; Curini, L.; Pallecchi, M.; Russo, E.; Niccolai, E.; Danza, G.; et al. Effect of ancient Khorasan wheat on gut microbiota, inflammation, and short-chain fatty acid production in patients with fibromyalgia. World J. Gastroenterol. 2022, 28, 1965–1980. [Google Scholar] [CrossRef]
- Minerbi, A.; Brereton, N.J.B.; Anjarkouchian, A.; Moyen, A.; Gonzalez, E.; Fitzcharles, M.A.; Shir, Y.; Chevalier, S. Dietary Intake Is Unlikely to Explain Symptom Severity and Syndrome-Specific Microbiome Alterations in a Cohort of Women with Fibromyalgia. Int. J. Environ. Res. Public. Health 2022, 19, 3254. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef]
- Longo, V.D.; Di Tano, M.; Mattson, M.P.; Guidi, N. Intermittent and periodic fasting, longevity and disease. Nat. Aging 2021, 1, 47–59. [Google Scholar] [CrossRef]
- Michalsen, A. Prolonged fasting as a method of mood enhancement in chronic pain syndromes: A review of clinical evidence and mechanisms. Curr. Pain. Headache Rep. 2010, 14, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.M.; Dell’Oro, M.; Kessler, C.S.; Schumann, D.; Steckhan, N.; Jeitler, M.; Fischer, J.M.; Spoo, M.; Kriegel, M.A.; Schneider, J.G.; et al. Efficacy of therapeutic fasting and plant-based diet in patients with rheumatoid arthritis (NutriFast): Study protocol for a randomised controlled clinical trial. BMJ Open 2021, 11, e047758. [Google Scholar] [CrossRef] [PubMed]
- Ring, R.M.; Eisenmann, C.; Kandil, F.I.; Steckhan, N.; Demmrich, S.; Klatte, C.; Kessler, C.S.; Jeitler, M.; Boschmann, M.; Michalsen, A.; et al. Mental and Behavioural Responses to Baha’i Fasting: Looking behind the Scenes of a Religiously Motivated Intermittent Fast Using a Mixed Methods Approach. Nutrients 2022, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Michalsen, A.; Li, C.; Kaiser, K.; Ludtke, R.; Meier, L.; Stange, R.; Kessler, C. In-Patient Treatment of Fibromyalgia: A Controlled Nonrandomized Comparison of Conventional Medicine versus Integrative Medicine including Fasting Therapy. Evid. Based Complement. Alternat Med. 2013, 2013, 908610. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi de Toledo, F.; Buchinger, A.; Burggrabe, H.; Holz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lutzner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting therapy—An expert panel update of the 2002 consensus guidelines. Forsch. Komplementmed 2013, 20, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Koppold, D.A.; Kandil, F.I.; Guttler, O.; Muller, A.; Steckhan, N.; Meiss, S.; Breinlinger, C.; Nelle, E.; Hartmann, A.M.; Jeitler, M.; et al. Effects of Prolonged Fasting during Inpatient Multimodal Treatment on Pain and Functional Parameters in Knee and Hip Osteoarthritis: A Prospective Exploratory Observational Study. Nutrients 2023, 15, 2695. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.S.; Jeitler, M.; Dhiman, K.S.; Kumar, A.; Ostermann, T.; Gupta, S.; Morandi, A.; Mittwede, M.; Stapelfeldt, E.; Spoo, M.; et al. Ayurveda in Knee Osteoarthritis-Secondary Analyses of a Randomized Controlled Trial. J. Clin. Med. 2022, 11, 3047. [Google Scholar] [CrossRef]
- Jeitler, M.; Michalsen, A.; Schwiertz, A.; Kessler, C.S.; Koppold-Liebscher, D.; Grasme, J.; Kandil, F.I.; Steckhan, N. Effects of a Supplement Containing a Cranberry Extract on Recurrent Urinary Tract Infections and Intestinal Microbiota: A Prospective, Uncontrolled Exploratory Study. J. Integr. Complement. Med. 2022, 28, 399–406. [Google Scholar] [CrossRef]
- Jeitler, M.; Roth, S.; Steckhan, N.; Meier, L.; Koppold-Liebscher, D.A.; Kandil, F.I.; Ostermann, T.; Stange, R.; Kessler, C.S.; Brinkhaus, B.; et al. Therapeutic Phlebotomy in Patients with Grade 1 Hypertension: A Randomized-Controlled Trial. J. Integr. Complement. Med. 2022, 28, 530–539. [Google Scholar] [CrossRef]
- Michalsen, A.; Ludtke, R.; Cesur, O.; Afra, D.; Musial, F.; Baecker, M.; Fink, M.; Dobos, G.J. Effectiveness of leech therapy in women with symptomatic arthrosis of the first carpometacarpal joint: A randomized controlled trial. Pain 2008, 137, 452–459. [Google Scholar] [CrossRef]
- Hartmann, A.; Dell’Oro, M.; Spoo, M.; Fischer, J.; Steckhan, N.; Jeitler, M.; Häupl, T.; Kandil, F.; Michalsen, A.; Koppold-Liebscher, D.; et al. To eat or not to eat—An exploratory randomized controlled trial on fasting and plant-based diet in rheumatoid arthritis (NutriFast-study). Front. Nutr. 2022, 9, 1030380. [Google Scholar] [CrossRef] [PubMed]
- Koppold-Liebscher, D.; Kessler, C.S.; Steckhan, N.; Bahr, V.; Kempter, C.; Wischnewsky, M.; Hubner, M.; Kunz, B.; Paul, M.; Zorn, S.; et al. Short-term fasting accompanying chemotherapy as a supportive therapy in gynecological cancer: Protocol for a multicenter randomized controlled clinical trial. Trials 2020, 21, 854. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Bushmakin, A.G.; Cappelleri, J.C.; Zlateva, G.; Sadosky, A.B. Minimal clinically important difference in the fibromyalgia impact questionnaire. J. Rheumatol. 2009, 36, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Ariani, A.; Bazzichi, L.; Sarzi-Puttini, P.; Salaffi, F.; Manara, M.; Prevete, I.; Bortoluzzi, A.; Carrara, G.; Scirè, C.A.; Ughi, N.; et al. The Italian Society for Rheumatology clinical practice guidelines for the diagnosis and management of fibromyalgia Best practices based on current scientific evidence. Reumatismo 2021, 73, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Feijoo, F.; Samartin-Veiga, N.; Carrillo-de-la-Peña, M.T. Quality of life in patients with fibromyalgia: Contributions of disease symptoms, lifestyle and multi-medication. Front. Psychol. 2022, 13, 924405. [Google Scholar] [CrossRef] [PubMed]
- Aster, H.C.; Evdokimov, D.; Braun, A.; Uceyler, N.; Sommer, C. Analgesic Medication in Fibromyalgia Patients: A Cross-Sectional Study. Pain. Res. Manag. 2022, 2022, 1217717. [Google Scholar] [CrossRef] [PubMed]
- Kundakci, B.; Kaur, J.; Goh, S.L.; Hall, M.; Doherty, M.; Zhang, W.; Abhishek, A. Efficacy of nonpharmacological interventions for individual features of fibromyalgia: A systematic review and meta-analysis of randomised controlled trials. Pain 2022, 163, 1432–1445. [Google Scholar] [CrossRef]
- Saracoglu, I.; Akin, E.; Aydin Dincer, G.B. Efficacy of adding pain neuroscience education to a multimodal treatment in fibromyalgia: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2022, 25, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.D.; Wang, L.Y.; Zhang, Z.H.; Zhang, D.X.; Lin, X.W.; Meng, T.; Qi, F. Effect of Exercise Interventions on Health-Related Quality of Life in Patients with Fibromyalgia Syndrome: A Systematic Review and Network Meta-Analysis. J. Pain. Res. 2022, 15, 3639–3656. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A.; Duschek, S.; Montoro, C.I. The Link between Fibromyalgia Syndrome and Anger: A Systematic Review Revealing Research Gaps. J. Clin. Med. 2022, 11, 844. [Google Scholar] [CrossRef]
- Antunes, M.D.; Marques, A.P. The role of physiotherapy in fibromyalgia: Current and future perspectives. Front. Physiol. 2022, 13, 968292. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Galasso, L.; Mulè, A.; Ciorciari, A.; Fornasini, F.; Montaruli, A.; Roveda, E.; Esposito, F. Sleep and spa therapies: What is the role of balneotherapy associated with exercise? A systematic review. Front. Physiol. 2022, 13, 964232. [Google Scholar] [CrossRef] [PubMed]
- Sturman, S.; Killingback, C. Is there a dose response relationship between soft tissue manual therapy and clinical outcomes in fibromyalgia? J. Bodyw. Mov. Ther. 2020, 24, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Fogleman, C.; McKenna, K. Integrative Health Strategies to Manage Chronic Pain. Prim. Care 2022, 49, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.M. Chronic Musculoskeletal Pain: Nonpharmacologic, Noninvasive Treatments. Am. Fam. Physician 2020, 102, 465–477. [Google Scholar] [PubMed]
- Bruce, B.K.; Allman, M.E.; Rivera, F.A.; Wang, B.; Berianu, F.; Butendieck, R.R.; Calamia, K.T.; Hines, S.L.; Rummans, T.A.; Niazi, S.K.; et al. Intensive Multicomponent Fibromyalgia Treatment: A Translational Study to Evaluate Effectiveness in Routine Care Delivery. J. Clin. Rheumatol. 2021, 27, e496–e500. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.S.; Ostermann, T.; Meier, L.; Stapelfeldt, E.; Schütte, S.; Duda, J.; Michalsen, A. Additive Complex Ayurvedic Treatment in Patients with Fibromyalgia Syndrome Compared to Conventional Standard Care Alone: A Nonrandomized Controlled Clinical Pilot Study (KAFA Trial). Evid. Based Complement. Alternat Med. 2013, 2013, 751403. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Coggins, J.; Derham, S.; Russell, J.; Walsh, N.E.; Lenguerrand, E.; Palmer, S.; Cramp, F. A feasibility randomised controlled trial of a Fibromyalgia Self-management Programme for adults in a community setting with a nested qualitative study (FALCON). BMC Musculoskelet. Disord. 2022, 23, 656. [Google Scholar] [CrossRef]
- Michalsen, A.; Schneider, S.; Rodenbeck, A.; Ludtke, R.; Huether, G.; Dobos, G.J. The short-term effects of fasting on the neuroendocrine system in patients with chronic pain syndromes. Nutr. Neurosci. 2003, 6, 11–18. [Google Scholar] [CrossRef]
- Schmidt, S.; Stange, R.; Lischka, E.; Kiehntopf, M.; Deufel, T.; Loth, D.; Uhlemann, C. Uncontrolled clinical study of the efficacy of ambulant fasting in patients with osteoarthritis. Forsch. Komplementmed 2010, 17, 87–94. [Google Scholar] [CrossRef]
- Wilhelmi de Toledo, F.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar] [CrossRef] [PubMed]
- Finnell, J.S.; Saul, B.C.; Goldhamer, A.C.; Myers, T.R. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement. Altern. Med. 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Value | Enrolled Patients |
|---|---|---|
| Sex | Female | 168 (95.5%) |
| Male | 8 (4.5%) | |
| Age [Years] | M ± SD | 54.2 ± 9.8 |
| Age Group | 18–35 years | 7 (4.0%) |
| 36–50 years | 40 (22.7%) | |
| 51–65 years | 116 (65.9%) | |
| 66–80 years | 13 (7.4%) | |
| Marital Status | Single | 30 (17.0%) |
| Married | 107 (60.8%) | |
| Separated/Divorced | 30 (17.0%) | |
| Widowed | 7 (4.0%) | |
| Other | 2 (1.1%) | |
| Household | Single | 43 (24.4%) |
| With Partner | 82 (46.6%) | |
| Single with Children | 13 (7.4%) | |
| With Partner and Children | 36 (20.5%) | |
| Other | 2 (1.1%) | |
| Schooling | Primary Schooling | 8 (4.5%) |
| Secondary Schooling | 91 (51.7%) | |
| High School | 22 (12.5%) | |
| University Degree | 51 (29.0%) | |
| Other | 4 (2.3%) | |
| Occupation | Self-employed | 7 (4.0%) |
| Civil Servant | 3 (1.7%) | |
| Employed | 83 (47.2%) | |
| Worker | 6 (3.4%) | |
| Homemaker | 9 (5.1%) | |
| Unemployed | 13 (7.4%) | |
| Retired | 28 (15.9%) | |
| Permanently Disabled | 20 (11.4%) | |
| Student | 2 (1.1%) | |
| Other | 5 (2.8%) | |
| Gross Salary | <EUR 20,000 | 87 (49.4%) |
| EUR 20,000–EUR 40,000 | 58 (33.0%) | |
| EUR 40,000–EUR 60,000 | 25 (14.2%) | |
| EUR 60,000–EUR 80,000 | 4 (2.3%) | |
| >EUR 80,000 | 2 (1.1%) | |
| Duration of Unemployment | Up to 3 Months | 71 (40.3%) |
| 3–6 Months | 45 (25.6%) | |
| 6 Months and Longer | 12 (6.8%) | |
| Not unemployed | 48 (27.3%) | |
| Diabetes | Present | 5 (2.8%) |
| Subjective Physical Health Status | Mildly Impaired | 6 (3.4%) |
| Impaired | 98 (55.7%) | |
| Strongly Impaired | 72 (40.9%) | |
| Subjective Psychological Health Status | Not Impaired | 10 (5.7%) |
| Mildly Impaired | 44 (25.0%) | |
| Impaired | 87 (49.4%) | |
| Strongly Impaired | 35 (19.9%) | |
| Psychotherapy | None Thus Far | 34 (19.3%) |
| Earlier | 90 (51.1%) | |
| Currently | 52 (29.5%) | |
| Nature-Based Therapies | Familiar with Concept | 118 (67.0%) |
| Stay at This Clinic | First | 102 (58.0%) |
| Second | 38 (21.6%) | |
| Third | 18 (10.2%) | |
| Fourth | 18 (10.2%) | |
| Fasting Experience | Never | 88 (50.0%) |
| Once | 30 (17.0%) | |
| Twice | 22 (12.5%) | |
| Three times | 15 (8.5%) | |
| Four times | 6 (3.4%) | |
| Five or more times | 15 (8.5%) | |
| Medication | Opioids | 6 |
| Pain Medication | 161 | |
| Neuropathic Medication | 1 | |
| Biologicals/MTX/Immuno-suppressants | 4 | |
| Prednisolone/Corticoids/ Prednisone | 4 | |
| Herbal Remedies | 39 | |
| Subjective Impairment by Main Symptomatology | NRS Scale [0–10]: M ± SD | 6.7 ± 1.3% |
| Anticipation of Inpatient Treatment Efficacy | NRS Scale [0–10]: M ± SD | 6.0 ± 2.0% |
| Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Visit | M | SD | n | M | SD | T | p | d |
| FIQ Total | V0 | 58.3 | 11.07 | 121 | |||||
| V1 | 44.6 | 15.5 | 121 | −13.7 | 13.92 | 10.8 | <0.0001 | 1.02 | |
| V2 | 49.2 | 15.47 | 85 | −8 | 14.24 | 5.15 | <0.0001 | 0.58 | |
| V3 | 51.8 | 15.18 | 77 | −5.1 | 15.81 | 2.79 | 0.0067 | 0.36 | |
| V4 | 50.9 | 15.11 | 63 | −4.6 | 14.44 | 2.5 | 0.0152 | 0.33 | |
| FIQ Subdomain 1 Function | V0 | 3.5 | 1.69 | 121 | |||||
| V1 | 3.2 | 2.01 | 121 | −0.3 | 1.65 | 2.16 | 0.0328 | 0.17 | |
| V2 | 3.7 | 2.36 | 101 | 0.4 | 2.46 | 1.48 | 0.1434 | 0.17 | |
| V3 | 3.8 | 2.41 | 91 | 0.4 | 2.4 | 1.67 | 0.099 | 0.2 | |
| V4 | 3.7 | 2.07 | 72 | 0.2 | 1.73 | 1.04 | 0.2998 | 0.11 | |
| FIQ Subdomain 2 Overall | V0 | 15.0 | 4.2 | 121 | |||||
| V1 | 10.9 | 5.22 | 121 | −4.1 | 5.43 | 8.35 | <0.0001 | 0.87 | |
| V2 | 13.6 | 4.52 | 85 | −0.8 | 5.42 | 1.42 | 0.1589 | 0.19 | |
| V3 | 14.3 | 3.97 | 77 | 0.1 | 5.04 | 0.19 | 0.8476 | 0.03 | |
| V4 | 13.3 | 4.26 | 63 | −0.6 | 5.55 | 0.9 | 0.3704 | 0.15 | |
| FIQ Subdomain 3 Symptoms | V0 | 39.8 | 9.03 | 121 | |||||
| V1 | 30.5 | 11.48 | 121 | −9.3 | 10.46 | 9.54 | <0.0001 | 0.89 | |
| V2 | 33.9 | 12.51 | 101 | −5.9 | 10.92 | 5.4 | <0.0001 | 0.53 | |
| V3 | 35.2 | 12.14 | 91 | −4.2 | 12.62 | 3.19 | 0.002 | 0.39 | |
| V4 | 35.1 | 11.74 | 72 | −3.3 | 11.21 | 2.5 | 0.0148 | 0.31 | |
| FIQ Pain (NRS) | V0 | 6.8 | 1.86 | 121 | |||||
| V1 | 5.7 | 2.62 | 121 | −1.1 | 2.54 | 4.85 | <0.0001 | 0.49 | |
| V2 | 5.7 | 2.49 | 101 | −1 | 2.44 | 4.27 | <0.0001 | 0.47 | |
| V3 | 5.9 | 2.53 | 91 | −0.7 | 2.35 | 2.75 | 0.0072 | 0.31 | |
| V4 | 6.1 | 2.18 | 72 | −0.6 | 2.14 | 2.41 | 0.0186 | 0.29 | |
| WHO-5 | V0 | 7.3 | 4.16 | 176 | |||||
| V1 | 12.5 | 5.12 | 142 | 5 | 4.87 | 12.25 | <0.0001 | 1.08 | |
| V2 | 11.1 | 5.28 | 101 | 3.6 | 5.47 | 6.66 | <0.0001 | 0.75 | |
| V3 | 10.5 | 5.29 | 91 | 2.5 | 5.86 | 4.08 | 0.0001 | 0.51 | |
| V4 | 10.8 | 5.05 | 72 | 2.6 | 5.21 | 4.14 | 0.0001 | 0.53 | |
| MAAS | V0 | 3.4 | 0.85 | 176 | |||||
| V1 | 3.7 | 0.9 | 142 | 0.3 | 0.72 | 5.46 | <0.0001 | 0.38 | |
| V2 | 3.4 | 0.94 | 101 | 0 | 0.75 | 0.55 | 0.5825 | 0.05 | |
| V3 | 3.4 | 0.86 | 91 | 0 | 0.84 | 0.36 | 0.7184 | 0.04 | |
| V4 | 3.5 | 0.87 | 72 | 0 | 0.82 | 0.04 | 0.966 | 0 | |
| HADS Depression | V0 | 13 | 4.07 | 176 | |||||
| V1 | 10.2 | 3.8 | 142 | −2.7 | 2.98 | 10.66 | <0.0001 | 0.68 | |
| V2 | 12.1 | 4.12 | 101 | −0.8 | 3.68 | 2.15 | 0.0338 | 0.2 | |
| V3 | 12.6 | 4.23 | 91 | 0 | 4.03 | 0.05 | 0.9588 | 0.01 | |
| V4 | 12.3 | 4.09 | 72 | −0.2 | 4.36 | 0.32 | 0.7481 | 0.04 | |
| HADS Anxiety | V0 | 12.9 | 4.02 | 176 | |||||
| V1 | 10 | 3.88 | 142 | −2.9 | 3.53 | 9.82 | <0.0001 | 0.73 | |
| V2 | 12 | 4.12 | 101 | −1 | 3.16 | 3.04 | 0.003 | 0.23 | |
| V3 | 11.8 | 4.13 | 91 | −0.8 | 3.08 | 2.5 | 0.0141 | 0.2 | |
| V4 | 11.9 | 4.17 | 72 | −1 | 3.78 | 2.32 | 0.0231 | 0.25 | |
| ASKU | V0 | 3.7 | 0.7 | 176 | |||||
| V1 | 3.8 | 0.63 | 142 | 0.1 | 0.57 | 1.8 | 0.0746 | 0.13 | |
| V2 | 3.5 | 0.85 | 101 | −0.2 | 0.86 | 2.82 | 0.0057 | 0.31 | |
| V3 | 3.4 | 0.89 | 91 | −0.4 | 0.86 | 4.5 | <0.0001 | 0.52 | |
| V4 | 3.5 | 0.9 | 72 | −0.3 | 0.83 | 2.72 | 0.0083 | 0.32 |
| Differences | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Visit | M | SD | n | M | SD | T | p | d |
| Cholesterol [mg/dL] | V0 | 237.2 | 43.75 | 102 | |||||
| V1 | 202 | 50.1 | 79 | −36.9 | 35.28 | 9.23 | <0.0001 | 0.81 | |
| LDL [mg/dL] | V0 | 150.9 | 38.42 | 97 | |||||
| V1 | 133 | 41.45 | 70 | −20.5 | 30.08 | 5.67 | <0.0001 | 0.53 | |
| HDL [mg/dL] | V0 | 62.6 | 13.31 | 97 | |||||
| V1 | 52.2 | 12.24 | 70 | −11.7 | 8.58 | 11.35 | <0.0001 | 0.91 | |
| Triglycerides [mg/dL] | V0 | 119.6 | 51.32 | 102 | |||||
| V1 | 106.5 | 26.51 | 78 | −11.5 | 42.26 | 2.39 | 0.0194 | 0.28 | |
| Weight [kg] | V0 | 82.7 | 17.72 | 161 | |||||
| Day 1 | 81.9 | 17.56 | 161 | ||||||
| V1 | 78.5 | 16.56 | 161 | −3.5 | 1.97 | 22.36 | <0.0001 | 0.2 | |
| Systolic BP [mmHg] | V0 | 126.3 | 14.56 | 161 | |||||
| V1 | 119.6 | 15.9 | 161 | −6.7 | 17.3 | 4.88 | <0.0001 | 0.44 | |
| Diastolic BP [mmHg] | V0 | 77.9 | 9.33 | 161 | |||||
| V1 | 74.3 | 8.82 | 161 | −3.7 | 9.92 | 4.67 | <0.0001 | 0.4 | |
| Discontinued | Reduced | Reduced to On-Demand | Unchanged | Increased to On-Demand | Increased | |
|---|---|---|---|---|---|---|
| Opioids | 0 | 2 | 0 | 3 | 0 | 1 |
| NSAIDs | 8 | 31 | 1 | 112 | 0 | 9 |
| Neuropathic Medication | 0 | 1 | 0 | 0 | 0 | 0 |
| Biologicals/MTX/Immunosuppressants | 0 | 0 | 0 | 3 | 0 | 1 |
| Prednisolone/Corticoids/ Lodotra | 0 | 2 | 0 | 2 | 0 | 0 |
| Herbal Remedies | 1 | 0 | 1 | 1 | 20 | 16 |
| Side Effect | Data from Recall Questionnaire | Extracted from Patient Records |
|---|---|---|
| Headache | 88 (62.0%) | 69 (50.0%) |
| Migraine | 19 (13.4%) | 4 (2.9%) |
| Mood Disturbance | 42 (29.6%) | 7 (5.1%) |
| Sickness | 31 (21.8%) | 23 (16.7%) |
| Hunger | 50 (35.2%) | 12 (8.7%) |
| Stomachache | 21 (14.8%) | 2 (1.4%) |
| Other | 50 (35.2%) | 99 (71.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koppold, D.A.; Kandil, F.I.; Müller, A.; Güttler, O.; Steckhan, N.; Meiss, S.; Breinlinger, C.; Nelle, E.; Rajput Khokhar, A.; Jeitler, M.; et al. Effects of Prolonged Medical Fasting during an Inpatient, Multimodal, Nature-Based Treatment on Pain, Physical Function, and Psychometric Parameters in Patients with Fibromyalgia: An Observational Study. Nutrients 2024, 16, 1059. https://doi.org/10.3390/nu16071059
Koppold DA, Kandil FI, Müller A, Güttler O, Steckhan N, Meiss S, Breinlinger C, Nelle E, Rajput Khokhar A, Jeitler M, et al. Effects of Prolonged Medical Fasting during an Inpatient, Multimodal, Nature-Based Treatment on Pain, Physical Function, and Psychometric Parameters in Patients with Fibromyalgia: An Observational Study. Nutrients. 2024; 16(7):1059. https://doi.org/10.3390/nu16071059
Chicago/Turabian StyleKoppold, Daniela A., Farid I. Kandil, Anna Müller, Oliver Güttler, Nico Steckhan, Sara Meiss, Carolin Breinlinger, Esther Nelle, Anika Rajput Khokhar, Michael Jeitler, and et al. 2024. "Effects of Prolonged Medical Fasting during an Inpatient, Multimodal, Nature-Based Treatment on Pain, Physical Function, and Psychometric Parameters in Patients with Fibromyalgia: An Observational Study" Nutrients 16, no. 7: 1059. https://doi.org/10.3390/nu16071059
APA StyleKoppold, D. A., Kandil, F. I., Müller, A., Güttler, O., Steckhan, N., Meiss, S., Breinlinger, C., Nelle, E., Rajput Khokhar, A., Jeitler, M., Hanslian, E., Fischer, J. M., Michalsen, A., & Kessler, C. S. (2024). Effects of Prolonged Medical Fasting during an Inpatient, Multimodal, Nature-Based Treatment on Pain, Physical Function, and Psychometric Parameters in Patients with Fibromyalgia: An Observational Study. Nutrients, 16(7), 1059. https://doi.org/10.3390/nu16071059