1. Introduction
Vitamin D is established to be essential for musculoskeletal health. However, older adults are prone to a low vitamin D status [
1]. As there is a growing population of >65-year-olds living in developed countries, including in the UK, where almost a quarter (24.4%) of the population is aged 60 or above [
2,
3], it is imperative to better understand the functional aspects of ageing skin. The early stage of vitamin D
3 metabolism is one such crucial area for translational research.
Skin contains the precursor for vitamin D
3 (cholecalciferol) synthesis, i.e., 7-dehydrocholesterol (7DHC), with its exposure to solar ultraviolet radiation (UVR) initiating vitamin D
3 biosynthesis. This is usually the major source of vitamin D in humans, with oral intake usually making a smaller contribution [
4]. Although both vitamin D
2 and vitamin D
3 can be obtained orally, only vitamin D
3 is synthesised by the skin. The 7DHC is found in higher concentrations in the upper layer of the skin, i.e., the epidermis, than the lower layer, i.e., the dermis, and is present in both keratinocytes and fibroblasts [
5,
6]. It is rapidly photoconverted to pre-vitamin D
3, with peak production in the ultraviolet B (UVB) waveband at 295 nm [
7]. Subsequently, pre-vitamin D
3 is converted more slowly to vitamin D
3 through heat-induced isomerisation. The vitamin D
3 diffuses through the dermal capillary beds and enters the body’s general circulation [
8]; hence, it is the metabolite that links the skin and blood pathways of vitamin D
3 supply and metabolism. It then undergoes hepatic hydroxylation to 25-hydroxyvitamin D
3 (25(OH)D
3), and following this, renal hydroxylation to the active hormone, i.e., 1,25-dihydroxyvitamin D
3 (1,25(OH)
2D
3). Ex vivo studies indicate that less than 15% of 7DHC is converted to pre-vitamin D
3 by a single low UVR exposure [
9]. However, higher levels of UVR exposure, and exposure to the ultraviolet A (UVA) component of solar UVR, can, on the other hand, lead to the production of inactive photoisomers, and if prolonged, might degrade newly formed vitamin D
3 [
9].
A study published in the 1980s involving skin specimens from surgical patients aged 8–92 years reported a negative correlation (r = −0.89) between patient age and epidermal 7DHC concentration, and concluded that a lower vitamin D status in older people could be explained by a lower skin 7DHC concentration [
10]. However, the study had some limitations, including the use of skin samples taken opportunistically from different body sites following a variety of surgical procedures; these ranged from leg amputation to reduction surgery and correction of protuberant ears in children [
10]. In contrast, a further study using skin from surgical samples and dermatology outpatients (from unspecified body sites) reported no correlation between patient age and skin 7DHC concentration [
11]. These earlier studies used liquid chromatography (LC)–UV detection methodology, and no further research addressing skin 7DHC concentration and ageing has to our knowledge been published.
Here, we have performed a standardised comparative study in prospectively recruited groups of younger and older healthy ambulant adults, with the objective firstly to compare the baseline skin content of 7DHC between these age groups, and secondly to examine their 7DHC–vitamin D
3 response to UVR exposure. Multiple sampling was performed of matched skin sites (for 7DHC) and blood (primarily for vitamin D
3) pre and post low, sub-erythemal (sub-sunburn) dose of solar simulated UVR (SSR; emission comprising 5% UVB and 95% UVA) in vivo. This replicates the dose and UVB: UVA balance that can be obtained from ambient solar UVR exposure in everyday life. A modern HPLC–MS/MS assay was employed for analyte measurements, including our recently developed methodology for measurement of 7DHC [
12,
13].
2. Materials & Methods
2.1. Volunteers
This study was performed in volunteers residing in Greater Manchester, UK, in the Photobiology Unit, Dermatology Research Centre, University of Manchester, based at Salford Royal Hospital, Greater Manchester, UK, in 2019. Inclusion criteria were healthy volunteers, ambulant males or females, of phototype I–III (white Caucasian), and aged 18–40 or 65–89 years. Exclusion criteria were history of skin cancer or photosensitivity, use of a sunbed or sunbathing within the past 3 months, and taking photoactive or bone active therapies, vitamin D > 200 IU (5 μg)/day, or anticoagulants (including aspirin, clopidogrel, warfarin, and propranolol).
Volunteers were recruited through advertisement and the Photobiology Unit database. Four potential volunteers were excluded: three younger adults did not fit the phototype criteria, and one older adult was undergoing immune-suppressant therapy. The North West Greater Manchester Research Ethics Committee gave approval (reference 18/NW/0493) on 25 July 2018, and volunteers completed the study during January–March 2019. The study adhered to Declaration of Helsinki principles, and volunteers gave written, informed consent. The trial is registered on the ISRCTN website, reference ISRCTN72674753.
2.2. Protocol
The study protocol is shown in a flowchart (
Figure 1), with further details of the methodology in the following text.
2.3. Phototype Assessment
Volunteers were assessed for sun-reactive skin type (phototype) by researchers based at the Photobiology Unit, Salford Royal Hospital, using a standardised series of questions relating to their history of skin sunburn and tanning responses to sunlight exposure, alongside their physical characteristics, including eye, hair, and skin colour (modified Fitzpatrick system [
14]).
2.4. Simulated Summer Sunlight Exposures
Volunteers were given a single sub-sunburn SSR dose: 1.3 standard erythemal dose (SED), equivalent to ~15 min UK midday summer exposure. This dose took approximately 6 min 20 s to administer.
A whole-body irradiation cabinet (Philips HB588 Sunstudio, Eindhoven, The Netherlands) was re-fitted with Arimed B fluorescent lamps (Cosmedico GmbH, Stuttgart, Germany), providing UVR emission close to UK ambient midday summer sunlight (290–400 nm; 95% UVA: 320–400 nm, 5% UVB: 290–320 nm). The cabinet emission was characterised with the use of a DTM300 spectroradiometer (Bentham, Reading, UK).
During the UVR exposure, the volunteers lay prone and wore standardised knee-length shorts and a short-sleeved T-shirt, i.e., exposing their hands, forearms, face, and lower legs, equating to ~35% of the body surface area (BSA). A 10 cm × 10 cm area was cut out from one side of their shorts to expose an upper buttock site (UVR biopsy site), and the other buttock was further covered with opaque UVR material (unexposed site). The volunteers wore protective eye goggles.
The study was performed as planned between the months of January and March, in accordance with ambient UVB being insufficient to produce appreciable vitamin D
3 at the study latitude (Manchester, UK; 53.5° N) during that time period. The UV index (UVI, measure of erythemal effective UV radiation) has been monitored in Manchester since 1997, and daily plots of the UVI can be viewed at
https://uk-air.defra.gov.uk/data/uv-index-graphs (accessed on 1 February 2020), where a bell-shaped curve indicates a clear day, and interruptions from such are indicative of clouds. When the UVI is less than 2, it is generally accepted as “safe,” i.e., minimal risk of sunburn and sun protection not required. Vitamin D synthesis is also deemed negligible in any practical scenario of daily life. The UVI only approaches 2 in the middle of the day at the very end of March, and earlier in the year it is far lower.
2.5. Skin Biopsies and 7DHC Concentration Analysis
Six 5 mm buttock skin punch biopsies were taken from each volunteer, i.e., two from unexposed skin, two immediately post-UVR (within 30 min of the irradiation), and two at 24 h post-UVR. Extraction, chromatographic separation, and measurement of 7DHC in µg was performed using HPLC–MS/MS, as previously reported [
12]. In brief, 7DHC was extracted using ethyl acetate:methanol (1:1,
v/
v) and derivatized with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) to enhance the ionization efficiency for electrospray ionization mass spectrometry (ESI–MS). Additionally, solid-supported liquid extraction (SLE) was employed to eliminate larger lipids from the 7DHC and reduce potential matrix effects. The LC–MS/MS assay met the validation criteria set by the International Council for Harmonisation. The calibration curve demonstrated linearity, with an average r2 value of 0.997. The coefficients of variation were 11.1% and 4.32% for inter-assay and intra-assay precision, respectively. The lower limit of quantification was 1.6 µg/g, and the upper limit was 100 µg/g. The average recovery of 7DHC through SLE was 91.4%.
Total cellular protein content of the biopsies was used to normalise the 7DHC assay results. This was performed with a colorimetric Lowry-based protein assay using the DC Protein Assay Kit II (Bio-Rad, Hercules, CA, USA). Samples and the calibration curve were prepared following the manufacturer’s protocol [
15], and the absorbance was measured using a Multiskan FC plate reader (Thermo Fisher Scientific, Waltham, MA, USA) with an internal shaker at 650 nm. The data obtained from the protein assay were expressed as mg of protein per biopsy, and the 7DHC concentration was expressed as µg of 7DHC per mg of protein.
2.6. Blood Sampling, Analysis of Serum Vitamin D Metabolites, and Routine Biochemistry
Blood samples were collected on 3 occasions, i.e., immediately prior to UVR, and 24 h and 7 days post-UVR, and serum was stored at −80 °C prior to analysis. Analysis of serum vitamin D
3 and vitamin D
2, 25(OH)D
2, and 25(OH)D
3 was performed by LC–MS/MS. Serum 25(OH)D
2 and 25(OH)D
3 were measured using a Micromass Quattro Ultima Pt electrospray ionisation mass spectrometer (Waters Corp., Milford, MA, USA), as described previously [
13]. The measurement ranges of the assays were 0.1–200 nmol/L for 25(OH)D
2 and 25(OH)D
3, calibrated using standard reference material SRM972a from the National Institute of Science and Technology (NIST). The mean coefficient of variation (CV) for intra-assay imprecision across the measuring range of the assays was 4.9% for 25(OH)D
3 and 8.3% for 25(OH)D
3, and the cumulative inter-assay CV was ≤7.4% for 25(OH)D
2 and ≤9.6% for 25(OH)D
3. Our 25(OH)D assays showed <6% accuracy bias against the Centers for Disease Control and Prevention’s reference method on the Vitamin D External Quality Assessment Scheme (DEQAS). We met the certification performance standards set by DEQAS throughout the time the analyses were performed. Vitamin D
3 and vitamin D
2 were analysed using the 4-phenyl-1,2,4-triazoline-3,5h-dione (PTAD) derivatised precursor to product mass ion transitions for vitamin D
3 (591.4 > 298.1) and vitamin D
2 (603.5 > 298.1). Serum vitamin D
3 and D
2 assays were calibrated using certified pure standards (IsoSciences, King of Prussia, PA, USA) spiked into vitamin D depleted serum (BBSI solutions, Recklinghausen, Germany) and an isotopic-labelled vitamin D
3-[23,24,25,26,27-13C5] as an internal standard (IsoSciences). The intra- and inter-assay precision coefficients of variation (CV) were 2.2–9.4% across the calibration range of 0–250 nmol/L, with an assay recovery of between 99.7 and 106.3%. The lower limit of quantification (LLoQ) was 1 nmol/L for both vitamin D
3 and vitamin D
2; the lower limit of detection (LLoD) was 0.5 nmol/L. All analyses were undertaken by the Good Clinical Laboratory Practice and DEQAS-certified Bioanalytical Facility at the University of East Anglia. Deficient vitamin D status was defined as 25(OH)D < 25 nmoL/L and sufficient status as 50 to 120 nmol/L [
16].
Routine biochemistry, including renal function and parathyroid hormone (PTH), was analysed at Salford Royal Hospital, Greater Manchester, UK. Analysis of PTH was with a 2-site sandwich immunoassay using direct chemiluminometric technology (Siemens Centaur XP Intact PTH assay), and routine biochemistry was analysed via the Siemens ADVIA assay.
2.7. Sample Size and Statistical Analyses
Sample size was constrained by the number of adults willing to undergo studies involving multiple skin biopsies. The sample size was estimated using confidence interval methodology. Based on data on 7DHC concentration in earlier studies [
10,
11], a sample size of n = 10 in each age group was estimated to have sufficient power to detect an approximate 2-fold difference in baseline skin 7DHC content between older and younger adults (80% power, alpha = 0.05). With respect to serum vitamin D
3, using published data [
17,
18], we estimated n = 10 participants in each age group would give 80% power to detect a mean difference between younger and older groups of approximately 17 nmol/L and 12 nmol/L at 24 h and 7 days post-UVR, respectively, at a 5% significance level.
The 7DHC data were transformed using the y = 1/y equation, after which the data were normally distributed and parametric tests were performed. Vitamin D3 results were assessed for normality using the Shapiro–Wilk test and QQ plots (data were normally distributed); similarly, 25(OH)D3 data were assessed for normality using the Shapiro–Wilk test and QQ plots. Data were analysed using mixed-effects analysis and the multiple comparisons (Sidak correction) test using GraphPad Prism statistical software (version 8.4.3, 10 June 2020).
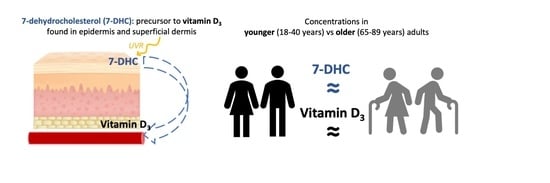
4. Discussion
This study was performed to explore whether the cutaneous portion of the vitamin D
3 biosynthetic pathway differs between younger and older adults. Under standardised conditions, and following our development of an updated skin 7DHC assay [
12], we showed that skin 7DHC concentration is not lower in older adults (mean 0.25 µg/mL) than younger adults (0.22 µg/mL), contrasting with a previous study of influence of age on 7DHC [
10]. We additionally examined the impact of low-dose SSR in vivo on skin 7DHC and serum vitamin D
3, i.e., the metabolite bridging the skin and the circulation in the vitamin D biosynthetic pathway. No detectable change occurred in 7DHC concentration of biopsies taken at two timepoints following UVR exposure, potentially reflecting the small percentage of 7DHC converted to pre-vitamin D, while serum vitamin D
3 increased at 24 h following UVR exposure in both groups. Consistent with this, there was a significant increase in vitamin D status (25(OH)D
3) from baseline to 24 h following UVR in the combined groups. Our findings indicate that baseline skin 7DHC concentration is not a limiting factor for vitamin D
3 synthesis in healthy older adults relative to younger adults, and that response to low-dose UVR is similar in older and younger adults.
The data collected in the current study indicated that skin 7DHC concentration was very similar in healthy volunteers in younger and older groups at baseline, and indeed under all conditions examined (unexposed skin, skin immediately post-UVR, and at 24 h post-UVR). This is in contrast with data reported by MacLaughlin and Holick in 1985, where an approximately two-fold lower baseline epidermal 7DHC content was found in surgical patients 70 years of age versus 30 years of age [
10]. Their findings could be attributable to the widely differing skin sites sampled between patients and age groups, and also potentially to the suboptimal health status of some tissue [
10,
16]. Additionally, the skin 7DHC content was normalised using the surface area and wet weight of samples, which can be unreliable due to potential water loss by evaporation; normalisation by dry weight [
20] or protein content [
21,
22], as we have used, is a more consistent method. In a technical study evaluating a skin 7DHC quantification method [
11], a seven-fold variation was found in the 7DHC concentration (n = 25, 12.1–80.6 ug/g dry weight), while no correlation was seen with age. Additionally, both of the earlier studies used an LC–UV system to quantify the 7DHC, which can be challenging when compounds of similar molecular weights are found in the extracted mixture.
The amount of 7DHC that is converted to pre-vitamin D
3 after one UVR exposure appears to be relatively small based on in vitro and ex vivo studies [
23]. Thus, it was found that within 5 min of summer sunlight exposure (Boston, MA, USA, 42° N), 3% of 7DHC in methanol in vitro was converted to pre-vitamin D
3, and after 1 h, ~9% was converted. Inert isomers were formed from pre-vitamin D
3, which accumulated with time. Similarly, in ex vivo skin experiments, after 3 h of sunlight exposure, only 7% of 7DHC was converted to pre-vitamin D
3 [
23]. In skin specimens irradiated ex vivo with narrowband UVB (295 ± 5 nm; 0.15 J/cm
2), the conversion rate of 7DHC to pre-vitamin D
3 ranged from 8 to 17%, with the highest rates in the youngest patients, and at a rate of 24 to 33% if evaluating the epidermis separately; these higher conversion rates may reflect the artificially narrow UV waveband used [
10]. MacLaughlin and Holick [
10] also irradiated skin specimens from five patients ex vivo with 0.15 J/cm
2 UVB (295 ± 5 nm) and found that the production of pre-vitamin D from 7DHC in very young people (aged 8 years and 18 years) was two-fold higher than in the three older patients (aged 77 years, 77 years, and 82 years), with mean conversions of 31.9% and 25.8%, respectively. In contrast, our protocol involved UVR exposure of emission spectrum (UVB and UVA) and a dose mimicking ~15 min of midday summer sunlight exposure, and our in vivo approach allowed for the potential influence of blood flow, body temperature, and neighbouring tissues; this improved representation of biology may have contributed to any reduction in 7DHC being too small for detection, and/or the 7DHC may have been rapidly restored. Additionally, the number of post-UVR timepoints that could be subjected to skin biopsy was limited by ethical acceptability.
Changes in circulating vitamin D
3 following UVR exposure also reflect the early stage of vitamin D metabolism. There was no difference in the mean baseline value of circulating vitamin D
3 between age groups (both were 1.5 nmol/L), and vitamin D
3 was observed to increase in both age groups at 24 h after UVR, indicating the conversion of 7DHC to pre-vitamin D
3. Although vitamin D
3 increased by 107% and 67% in the younger and older groups, respectively, there was no statistically significant difference between groups. At present, there are no comparable published studies examining the influence of age on circulating vitamin D
3 response to UVR exposure. However, studies using a variety of protocols have examined the impact of UVR on the level of serum vitamin D
3, and our baseline measurements are similar to those of other recent studies reporting average levels of ~1.3 and 2.5 nmoL/L [
18]. Other studies generally show larger average increases in serum vitamin D
3 post-UVR [
17], which can be accounted for by protocol differences, including higher UVR dose, higher UVB content (converts 7DHC to pre-vitamin D
3) relative to UVA (in contrast, leads to production of inactive isomers), repeated exposures, and more BSA exposed. Thus, reported average increases in serum vitamin D
3 post-UVR have ranged from 6.5 nmoL/L in 15 adults aged 23 ± 2.3 years given a single UVR dose of 2.2 ± 0.8 SED to ~50% BSA [
18], to 37.7 nmol/L in 10 volunteers aged 48 ± 12 years given 0.66–1.4 SED on 3 consecutive days to ~50% BSA [
17]. However, similarly to Libon et al. [
18], who measured serum vitamin D
3 concentration 5 days post-UVR, the serum vitamin D
3 concentration in our study decreased towards baseline at 7 days post-UVR.
The strengths of our study include its prospective design, use of healthy volunteers, standardised procedures and skin sites, mimicking of casual natural sunlight exposure, use of modern assays, and originality of the approach. We recruited volunteers of matched skin type, and skin samples were collected from a body site protected from the sun. Furthermore, the study was conducted during winter months in the UK, when ambient UVB is insufficient to produce appreciable vitamin D
3 in skin. The UV irradiation protocol was designed to mimic natural sunlight exposure in humans, using UVR comprising 95% and 5% UVB (similar to ambient UVR in summer), approximately 35% BSA exposure (as when wearing summer clothes, such as T-shirt and shorts), and a low sub-erythemal UVR dose that is believed to be the most efficient in vitamin D
3 synthesis [
9]. We previously showed that such low doses of SSR can increase vitamin D status whilst minimising but not eliminating the risk of skin damage in lighter skin types [
24,
25].
A limitation of the study is the relatively low number of volunteers, as intensive in vivo multiple biopsy studies are challenging to recruit and perform in higher numbers, and this potentially resulted in missed detection of significant differences. Repeated UVR doses might also be needed to detect a difference in 7DHC concentration following UVR exposure. Similarly, repeated low doses of UVR, as well as a study with a larger volunteer sample size, are indicated to further explore serum vitamin D3 differences between age groups post-UVR exposure.
Taken together with inconsistent evidence of the benefits of vitamin D supplements for musculoskeletal health in vitamin D-replete healthy older adults [
26], our study may influence assumptions regarding oral vitamin D requirements [
27] in the >65-year-old age group. Our findings do not negate the need to be mindful of vitamin D requirements in older adults in residential care or with lower levels of independent mobility, as they can have significantly reduced sunlight exposure and hence lower vitamin D status [
28].