Human Milk Banking–Facts and Issues to Resolve
Abstract
:1. Introduction
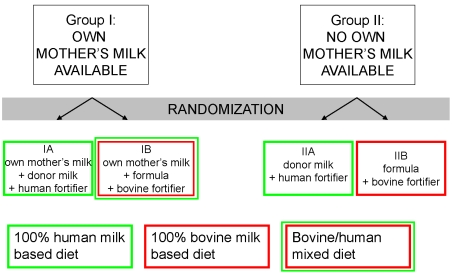
2. Evidence of the Benefits of Donor Milk
3. Studies to be Undertaken

4. Additional Issues to be Resolved
4.1. Method of Pasteurization
4.2. Safety of the Milk
4.3. Method of Fortification
References
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; Goulet, O.; Kalhan, S.C.; Kolacek, S.; Koletzko, B.; Lapillonne, A.; Mihatsch, W.; Moreno, L.; Neu, J.; Poindexter, B.; Puntis, J.; Putet, G.; Rigo, J.; Riskin, A.; Salle, B.; Sauer, P.; Shamir, R.; Szajewska, H.; Thureen, P.; Turck, D.; van Goudoever, J.B.; Ziegler, E.E. Eco Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar]
- Tully, D.B.; Jones, F.; Tully, M.R. Donor milk: what's in it and what's not. J. Hum. Lact. 2001, 17, 152–155. [Google Scholar]
- Bjorksten, B.; Burman, L.G.; De Chateau, P.; Fredrikzon, B.; Gothefors, L.; Hernell, O. Collecting and banking human milk: to heat or not to heat? Br. Med. J. 1980, 281, 765–769. [Google Scholar] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar]
- Quigley, M.A.; Henderson, G.; Anthony, M.Y.; McGuire, W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, CD002971. [Google Scholar]
- Hylander, M.A.; Strobino, D.M.; Dhanireddy, R. Human milk feedings and infection among very low birth weight infants. Pediatrics 1998, 102, E38. [Google Scholar]
- Narayanan, I.; Prakash, K.; Murthy, N.S.; Gujral, V.V. Randomised controlled trial of effect of raw and holder pasteurised human milk and of formula supplements on incidence of neonatal infection. Lancet 1984, 2, 1111–1113. [Google Scholar]
- de Silva, A.; Jones, P.W.; Spencer, S.A. Does human milk reduce infection rates in preterm infants? A systematic review. Arch. Dis. Child Fetal. Neonatal Ed. 2004, 89, F509–513. [Google Scholar]
- Lucas, A.; Morley, R.; Cole, T.J.; Gore, S.M. A randomised multicentre study of human milk versus formula and later development in preterm infants. Arch. Dis. Child Fetal. Neonatal Ed. 1994, 70, F141–146. [Google Scholar]
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Lucas, A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet 2004, 363, 1571–1578. [Google Scholar]
- Singhal, A.; Cole, T.J.; Lucas, A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet 2001, 357, 413–419. [Google Scholar]
- Flacking, R.; Wallin, L.; Ewald, U. Perinatal and socioeconomic determinants of breastfeeding duration in very preterm infants. Acta Paediatr. 2007, 96, 1126–1130. [Google Scholar]
- Victorino, C.C.; Gauthier, A.H. The social determinants of child health: variations across health outcomes - a population-based cross-sectional analysis. BMC Pediatr. 2009, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Orloff, S.L.; Wallingford, J.C.; McDougal, J.S. Inactivation of human immunodeficiency virus type I in human milk: effects of intrinsic factors in human milk and of pasteurization. J. Hum. Lact. 1993, 9, 13–17. [Google Scholar]
- Hamprecht, K.; Maschmann, J.; Muller, D.; Dietz, K.; Besenthal, I.; Goelz, R.; Middeldorp, J.M.; Speer, C.P.; Jahn, G. Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr. Res. 2004, 56, 529–535. [Google Scholar]
- Terpstra, F.G.; Rechtman, D.J.; Lee, M.L.; Hoeij, K.V.; Berg, H.; Van Engelenberg, F.A.; Van't Wout, A.B. Antimicrobial and antiviral effect of high-temperature short-time (HTST) pasteurization applied to human milk. Breastfeed Med. 2007, 2, 27–33. [Google Scholar]
- Vetrugno, V. Safety of milk and milk derivatives in relation to BSE: the lactoferrin example. Biometals 2004, 17, 353–356. [Google Scholar]
- Richardus, J.H.; Donkers, A.; Dumas, A.M.; Schaap, G.J.; Akkermans, J.P.; Huisman, J.; Valkenburg, H.A. Q fever in the Netherlands: a sero-epidemiological survey among human population groups from 1968 to 1983. Epidemiol. Infect. 1987, 98, 211–219. [Google Scholar]
- Cerf, O.; Condron, R. Coxiella burnetii and milk pasteurization: an early application of the precautionary principle? Epidemiol. Infect. 2006, 134, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawoger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; Laroia, N.; Ehrenkranz, R.A.; Dudell, G.; Cristofalo, E.A.; Meier, P.; Lee, M.L.; Rechtman, D.J.; Lucas, A. An Exclusively Human Milk-Based Diet Is Associated with a Lower Rate of Necrotizing Enterocolitis than a Diet of Human Milk and Bovine Milk-Based Products. J. Pediatr. 2010, 156, 562–567. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Corpeleijn, W.E.; Vermeulen, M.J.; Van Vliet, I.; Kruger, I.C.; Van Goudoever, J.B. Human Milk Banking–Facts and Issues to Resolve. Nutrients 2010, 2, 762-769. https://doi.org/10.3390/nu2070762
Corpeleijn WE, Vermeulen MJ, Van Vliet I, Kruger IC, Van Goudoever JB. Human Milk Banking–Facts and Issues to Resolve. Nutrients. 2010; 2(7):762-769. https://doi.org/10.3390/nu2070762
Chicago/Turabian StyleCorpeleijn, Willemijn E., Marijn J. Vermeulen, Ineke Van Vliet, I. Caroline Kruger, and Johannes B. Van Goudoever. 2010. "Human Milk Banking–Facts and Issues to Resolve" Nutrients 2, no. 7: 762-769. https://doi.org/10.3390/nu2070762
APA StyleCorpeleijn, W. E., Vermeulen, M. J., Van Vliet, I., Kruger, I. C., & Van Goudoever, J. B. (2010). Human Milk Banking–Facts and Issues to Resolve. Nutrients, 2(7), 762-769. https://doi.org/10.3390/nu2070762