Inclusion of Fermented Foods in Food Guides around the World
Abstract
:1. Introduction
2. Fermented Foods
| Fermented Food and Main Constituents | Country |
|---|---|
| Yogurt—milk, L. bulgaricus, S. thermophilus | Greece, Turkey |
| Kefir—milk, kefir grains, Saccharomyces cerevisiae and L. plantarum | Russia |
| Sauerkraut—green cabbage, L. plantarum | Germany |
| Kimchi—cabbage, Leuconostoc mesenteroides | South Korea |
| Cortido—cabbage, onions, carrots | El Salvador |
| Sourdough—flour, water, L. reuteri, Saccharomyces cerevisiae | Egypt |
| Kvass—beverage from black or rye bread, Lactobacillus | Russia |
| Kombucha—black, green, white, pekoe, oolong, or darjeeling tea, water, sugar, Gluconacetobacter and Zygosaccharomyces | Russia and China |
| Pulque—beverage from agave plant sap, Zymomonas mobilis | Mexico |
| Kaffir beer—beverage from kaffir maize, Lactobacillus sp. | South Africa |
| Ogi—cereal, Lactobacillus sp., Saccharomyces sp., Candida sp. | Africa |
| Igunaq—fermented walrus | Canada |
| Miso—soybeans, Aspergillus oryzae, Zygosaccharomyces, Pediococcus sp. | Japan |
| Tepa—Stinkhead fermented fish | USA |
| Dosa—fermented rice batter and lentils, L. plantarum | India |
| Cheddar and stilton cheeses—Penicillium roqueforti, Yarrowia lipolytica, Debaryomyces hansenii, Trichosporon ovoides | United Kingdom |
| Surströmming—fermented herring, brine, Haloanaerobium praevalens, Haloanaerobium alcaliphilum | Sweden |
| Crème fraîche—soured dessert cream, L. cremoris, L. lactis | France |
| Fermented sausage—Lactobacillus, Pediococcus, or Micrococcus | Greece and Italy |
| Wine—various organisms particularly Saccharomyces cerevisiae | Georgia |
3. Examples of Fermented Foods from around the World
| Food | Country | Average Annual Consumption (per Person) |
|---|---|---|
| Beer | Germany | 106 L |
| Cheese | UK | 10 kg |
| Kimchi | Korea | 22 kg |
| Miso | Japan | 7 kg |
| Soy Sauce | Japan | 10 L |
| Tempeh | Indonesia | 18 kg |
| Wine | Italy, Portugal | 90 L |
| Argentina | 70 L | |
| Finland | 40 L | |
| Yogurt | Netherlands | 25 L |
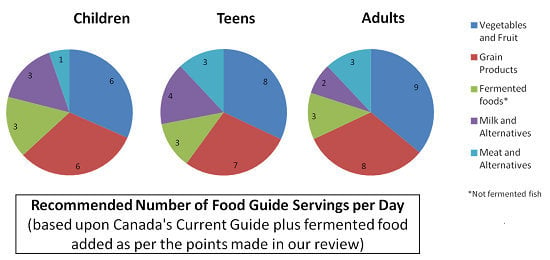
4. Nutritional Guides from around the World


5. Benefits of Fermented Foods
5.1. Benefits of Fermented Dairy Products
5.2. Benefits of Fermented Foods in Vulnerable Populations
5.3. Other Fermented Foods and Benefits
5.4. Adverse Effects of Fermented Foods
6. Conclusions and Recommendations
Author Contributions
Conflicts of Interest
References
- Various, A. Regimen for Health. In Hippocratic Writings; Lloyd, G.E.R., Chadwick, J., Mann, W.N., Eds.; Penguin Books Ltd.: London, UK, 1983; pp. 154–196. [Google Scholar]
- Canada’s Food Guides from 1942 to 1992. Available online: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/context/fg_history-histoire_ga-eng.php#fnb1 (accessed on 12 October 2014).
- Walker, P.M.B. Chambers Science and Technology Dictionary; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Available online: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf (accessed on 23 September 2014).
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; de Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lin, J.; Gong, D. Identification of lactic acid bacterial strains with high conjugated linoleic acid-producing ability from natural sauerkraut fermentations. J. Food Sci. 2009, 74, 154–158. [Google Scholar] [CrossRef]
- Carasi, P.; Diaz, M.; Racedo, S.M.; de Antoni, G.; Urdaci, M.C.; Serradell Mde, L. Safety characterization and antimicrobial properties of kefir-isolated Lactobacillus kefiri. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Reid, G. African traditional fermented foods and probiotics. J. Med. Food 2009, 12, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Lee, J.E.; Lee, C.H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Sicard, D.; Legras, J.L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. C. R. Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R. Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–602. [Google Scholar]
- Fermented Fruits and Vegetables: A Global Perspective. Available online: http://www.fao.org/docrep/x0560e/x0560e00.htm (accessed on 23 September 2014).
- Fermented Foods. Available online: http://www.nzifst.org.nz/myfiles/Expt_6.ppt (accessed on 23 September 2014).
- Pagoda Illustration. Available online: http://www.cnsoc.org/en/nutrition.asp?s=9&nid=806 (accessed on 23 September 2014).
- Dietary Guidelines for Indians: A Manual. Available online: http://ninindia.org/DietaryguidelinesforIndians-Finaldraft.pdf (accessed on 23 September 2014).
- Keszei, A.P.; Schouten, L.J.; Goldbohm, A.; van den Brandt, P.A. Dairy intake and the risk of bladder cancer in the Netherlands cohort study on diet and cancer. Am. J. Epidemiol. 2009, 171, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Sonedstedt, E.; Wirfält, E.; Wallstrom, P.; Gullberg, B.; Orho-Melander, M.; Hedblad, B. Dairy products and its association with incidence of cardiovascular disease: The Malmö diet and cancer cohort. Eur. J. Epidemiol. 2011, 26, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, A.R.; Christensen, L.B.; Holm-Pedersen, P.; Avlund, K.; Boucher, B.J.; Heitmann, B.L. Intake of dairy products in relation to periodontitis in older Danish adults. Nutrients 2012, 4, 1219–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, D.; Chattorai, D.K.; Chattopadhyay, P. Studies on changes in microstructure and proteolysis in cow and soy milk curd during fermentation using lactic cultures for improving protein bioavailability. J. Food Sci. Technol. 2013, 50, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, C.; Li, X.; Li, J.; Chu, R.; Wang, H. Higher dietary folate intake reduces the breast cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Morse, N.L. Benefits of docosahexaenoic acid, folic acid, vitamin D and iodine on foetal and infant brain development and function following maternal supplementation during pregnancy and lactation. Nutrients 2012, 4, 799–840. [Google Scholar] [CrossRef] [PubMed]
- Pitkala, K.H.; Strandberg, T.E.; Finne Soveri, U.H.; Ouwehand, A.C.; Poussa, T.; Salminen, S. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J. Nutr. Health Aging 2007, 11, 305–311. [Google Scholar] [PubMed]
- Tabbers, M.M.; Chmielewska, A.; Roseboom, M.G.; Crastes, N.; Perrin, C.; Reitsma, J.B.; Norbruis, O.; Szajewska, H.; Benninga, M.A. Fermented milk containing Bifidobacterium lactis DN-173 010 in childhood constipation: A randomized, double-blind, controlled trial. Pediatrics 2011, 127, 1392–1399. [Google Scholar] [CrossRef]
- Mazlyn, M.M.; Nagarajah, L.H.; Fatimah, A.; Norimah, A.K.; Goh, K.L. Effects of a probiotic fermented milk on functional constipation: A randomized, double-blind, placebo-controlled study. J. Gastroenterol. Hepatol. 2013, 28, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, H.; Lu, C.; Kang, Z.; Luo, Y.; Feng, L.; Lu, X. Fermented milk supplemented with probiotics and prebiotics can effectively alter the intestinal microbiota and immunity of host animals. J. Dairy Sci. 2012, 95, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Surono, I.S.; Koestomo, F.P.; Novitasari, N.; Zakaria, F.R. Novel probiotic Enterococcus faecium IS-27526 supplementation increased total salivary sIgA level and bodyweight of pre-school children: A pilot study. Anaerobe 2011, 17, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010, 103, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- De Vrese, M.; Winkler, P.; Rautenberg, P.; Harder, T.; Noah, C.; Laue, C.; Ott, S.; Hampe, J.; Schreiber, S.; Heller, K.; et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine 2006, 24, 6670–6674. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.; Heinemann, C.; Baroja, M.L.; Bruce, A.W.; Beuerman, D.; Madrenas, J.; Reid, G. Oral administration of the probiotic combination Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 for human intestinal applications. Int. Dairy J. 2002, 12, 191–196. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakai, S.; Tsukamoto, T.; Masumori, N.; Akaza, H.; Miyanaga, N.; Kitamura, T.; Kawabe, K.; Kotake, T.; Kuroda, M.; et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 2002, 68, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Narva, M.; Nevala, R.; Poussa, T.; Korpela, R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur. J. Nutr. 2004, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.; Noda, M.; Awaya, T.; Nomura, K.; Oku, H.; Sugiyama, M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Sharafedtinov, K.K.; Plotnikova, O.A.; Alexeeva, R.I.; Sentsova, T.B.; Songisepp, E.; Stsepetova, J.; Smidt, I.; Mikelsaar, M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—A randomized double-blind placebo-controlled pilot study. Nutr. J. 2013, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Peguet-Navarro, J.; Dezutter-Dambuyant, C.; Buetler, T.; Leclaire, J.; Smola, H.; Blum, S.; Bastien, P.; Breton, L.; Gueniche, A. Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double blind, randomized, placebo controlled clinical trial. Eur. J. Dermatol. 2008, 18, 504–511. [Google Scholar] [PubMed]
- Pérez, N.; Iannicelli, J.C.; Girard-Bosch, C.; González, S.; Varea, A.; Disalvo, L.; Apezteguia, M.; Pernas, J.; Vicentin, D.; Cravero, R.; et al. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio-economic status. Eur. J. Nutr. 2010, 49, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Baroja, M.L.; Kirjavainen, P.V.; Hekmat, S.; Reid, G. Anti-inflammatory effects of probiotic-yogurt in inflammatory bowel disease patients. Clin. Exp. Immunol. 2007, 149, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, D.; Penders, J.; Masclee, A.; Pierik, M. Probiotics in the management of inflammatory bowel disease: A systematic review of intervention studies in adult patients. Drugs 2012, 72, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Linsalata, M.; Notarnicola, M.; Tutino, V.; Russo, F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: The role of cellular polyamines. BMC Microbiol. 2014, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Persborn, M.; Gerritsen, J.; Wallon, C.; Carlsson, A.; Akkermans, L.M.; Söderholm, J.D. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 38, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Osazuwa, E.O.; Osadolor, B.E.; Bruce, A.W.; Reid, G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J. Clin. Gastroenterol. 2008, 42, 239–243. [Google Scholar] [PubMed]
- Irvine, S.L.; Hummelen, R.B.S.; Hekmat, S.; Looman, C.; Changalucha, J.; Habbema, D.F.; Reid, G. Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDS. J. Clin. Gastroenterol. 2010, 44, 201–205. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Hansen, S.; Boucsein, K.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of Lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation 2002, 74, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Rayes, N.; Seehofer, D.; Müller, A.R.; Hansen, S.; Bengmark, S.; Neuhaus, P. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery—Results of a prospective trial. Z. Gastroenterol. 2002, 40, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Rayes, N.; Hansen, S.; Seehofer, D.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of fiber and lactobacilli versus conventional nutrition: A controlled trial in patients with major abdominal surgery. Nutrition 2002, 18, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Huang, M.J.; Zhang, X.W.; Wang, L.; Huang, N.Q.; Peng, H.; Lan, P.; Peng, J.S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Kozer, E.; Lazarovitch, Z.; Broide, E.; Goldman, M. Probiotics and the immunological response to infant vaccinations: A prospective, placebo controlled pilot study. Arch. Dis. Child. 2011, 96, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Kuitunen, M.; Haahtela, T.; Korpela, R.; Poussa, T.; Savilahti, E. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr. Allergy Immunol. 2010, 21, 67–73. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Hammarström, M.L.; Hernell, O. Probiotics during weaning reduce the incidence of eczema. Pediatr. Allergy Immunol. 2009, 20, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Grüber, C.; Wendt, M.; Sulser, C.; Lau, S.; Kulig, M.; Wahn, U.; Werfel, T.; Niggemann, B. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007, 62, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Kirjavainen, P.V.; Fisher, C.; Anukam, K.; Summers, K.; Hekmat, S.; Reid, G. Development and pilot evaluation of a novel probiotic mixture for the management of seasonal allergic rhinitis. Can. J. Microbiol. 2010, 56, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Naghibi Rad, M.; Rahimi Foroushani, A.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.E.; Björck, I.M. A bilberry drink with fermented oatmeal decreases postprandial insulin demand in young healthy adults. Nutr. J. 2011, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Rizzello, C.G.; Alfonsi, G.; Arnault, P.; Cappelle, S.; di Cagno, R.; Gobbetti, M. Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br. J. Nutr. 2007, 98, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.M.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Dietary dairy product intake and incident type 2 diabetes: A prospective study using dietary data from a 7-day food diary. Diabetologia 2014, 57, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, N.; Burton, J.P.; Suppiah, P.; Reid, G.; Stebbings, S. The role of the microbiome in rheumatic diseases. Curr. Rheumatol. Rep. 2013, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Noto Llana, M.; Sarnacki, S.H.; Aya Castañeda Mdel, R.; Bernal, M.I.; Giacomodonato, M.N.; Cerquetti, M.C. Consumption of Lactobacillus casei fermented milk prevents Salmonella reactive arthritis by modulating IL-23/IL-17 expression. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Pineda Mde, L.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. 2011, 17, 347–354. [Google Scholar]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital admissions/visits associated with drug-drug interactions: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Budnitz, D.S.; Lovegrove, M.C.; Shehab, N.; Richards, C.L. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011, 365, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Plana, N.; Nicolle, C.; Ferre, R.; Camps, J.; Cos, R.; Villoria, J.; Masana, L.; DANACOL Group. Plant sterol-enriched fermented milk enhances the attainment of LDL-cholesterol goal in hypercholesterolemic subjects. Eur. J. Nutr. 2008, 47, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, X.L.; Sui, J.Z.; Chen, S.Y.; Xie, Y.T.; Deng, Y.; Wang, J.; Xie, L.; Li, T.J.; He, Y.; et al. Meta-analysis of randomized controlled trials on the efficacy of probiotics in Helicobacter pylori eradication therapy in children. Eur. J. Pediatr. 2014, 173, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Nagata, S. Beneficial microbes for premature infants, and children with malignancy undergoing chemotherapy. Benef. Microbes 2010, 1, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, M.; Fortier, N.; Guénette, S.; L’ecuyer, A.; Savoie, M.; Franco, M.; Lachaine, J.; Weiss, K. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Can. J. Gastroenterol. 2007, 21, 732–736. [Google Scholar] [PubMed]
- Wenus, C.; Goll, R.; Loken, E.B.; Biong, A.S.; Halvorsen, D.S.; Florholmen, J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur. J. Clin. Nutr. 2008, 62, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Glück, U.; Gebbers, J.O. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am. J. Clin. Nutr. 2003, 77, 517–520. [Google Scholar] [PubMed]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.O.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; et al. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. MeTable 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Ke, L.; Yu, P.; Zhang, Z.X. Novel epidemiologic evidence for the association between fermented fish sauce and esophageal cancer in South China. Int. J. Cancer 2002, 99, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Pignatelli, B.; Malaveille, C.; Bouvier, G.; Shuker, D.; Hautefeuille, A.; Zhang, R.F.; Bartsch, H. Levels of direct-acting mutagens, total N-nitroso compounds in nitrosated fermented fish products, consumed in a high-risk area for gastric cancer in southern China. Mutat. Res. 1992, 265, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Rabie, M.A.; Elsaidy, S.; el-Badawy, A.A.; Siliha, H.; Malcata, F.X. Biogenic amine contents in selected Egyptian fermented foods as determined by ion-exchange chromatography. J. Food Prot. 2011, 74, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.; Huch, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African fermented foods and probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Mathara, J.M.; Schillinger, U.; Kutima, P.M.; Mbugua, S.A.; Holzapfel, W.H. Isolation, Identification and characterization of the dominant microorganisms of kule naoto: The Maasai traditional fermented milk in Kenya. Int. J. Food Microbiol. 2004, 94, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tiwari, S.K. Probiotic potential of Lactobacillus plantarum LD1 isolated from batter of Dosa, a South Indian fermented food. Probiotics Antimicrob. Proteins 2014, 6, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.E.; Ngesa, A.; Onyang’o, J.; Alnwick, D.; Tomkins, A.M. Fermentation—A traditional anti-diarrhoeal practice lost? The use of fermented foods in urban and rural Kenya. Int. J. Food Sci. Nutr. 1996, 47, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.F.; Lin, W.T.; Chen, Y.L.; Huang, H.L.; Yang, W.Z.; Lee, C.Y.; Chen, M.H.; Wang, T.N.; Huang, M.C.; Chiu, Y.W.; et al. Elevated serum triglyceride and retinol-binding protein 4 levels associated with fructose-sweetened beverages in adolescents. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W.; Dumke, K.A.; Goran, M.I. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition 2014, 30, 928–935. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of Fermented Foods in Food Guides around the World. Nutrients 2015, 7, 390-404. https://doi.org/10.3390/nu7010390
Chilton SN, Burton JP, Reid G. Inclusion of Fermented Foods in Food Guides around the World. Nutrients. 2015; 7(1):390-404. https://doi.org/10.3390/nu7010390
Chicago/Turabian StyleChilton, Stephanie N., Jeremy P. Burton, and Gregor Reid. 2015. "Inclusion of Fermented Foods in Food Guides around the World" Nutrients 7, no. 1: 390-404. https://doi.org/10.3390/nu7010390
APA StyleChilton, S. N., Burton, J. P., & Reid, G. (2015). Inclusion of Fermented Foods in Food Guides around the World. Nutrients, 7(1), 390-404. https://doi.org/10.3390/nu7010390