Lipid Extract from Hard-Shelled Mussel (Mytilus coruscus) Improves Clinical Conditions of Patients with Rheumatoid Arthritis: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. HMLE Capsule and Corn Oil Capsule Preparation
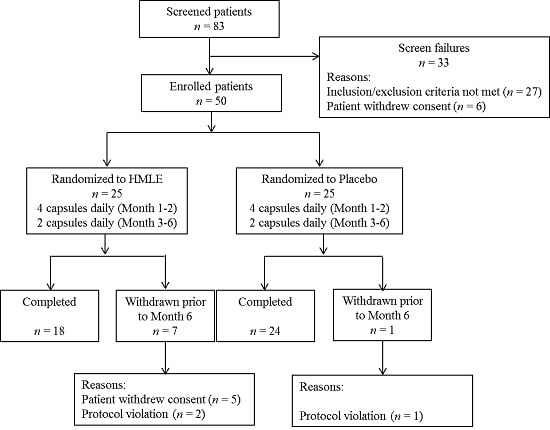
2.2. Subjects
2.3. Study Design
2.4. Laboratory Measurements and Clinical Evaluation
2.5. Statistical Analysis
3. Results
3.1. Lipid Profiles and Fatty Acids Composition of HMLE
| Fatty Acid | Separated from HMLE | Total HMLE | Placebo | |
|---|---|---|---|---|
| TAG | FFA | |||
| C14:0 | 3.92 ± 0.04 | 4.25 ± 0.04 | 2.71 ± 0.09 | ND |
| C15:0 | 0.85 ± 0.03 | 1.02 ± 0.01 | 0.81 ± 0.06 | ND |
| C16:0 | 29.00 ± 0.59 | 31.37 ± 0.46 | 24.83 ± 0.87 | 12.14 ± 0.48 |
| C17:0 | 1.58 ± 0.08 | 1.55 ± 0.03 | 2.24 ± 0.09 | ND |
| C18:0 | 4.45 ± 0.14 | 3.97 ± 0.06 | 6.31 ± 0.13 | 1.70 ± 0.11 |
| Total SFA | 39.80 ± 1.01 | 42.16 ± 0.98 | 36.90 ± 0.81 | 13.84 ± 0.41 |
| C16:1 | 4.08 ± 0.06 | 6.52 ± 0.06 | 7.28 ± 0.11 | ND |
| C17:1 | 0.23 ± 0.01 | 0.28 ± 0.05 | 3.10 ± 0.07 | ND |
| C18:1n-9 | 1.08 ± 0.08 | 1.07 ± 0.04 | 1.65 ± 0.08 | 29.89 ± 0.72 |
| C18:1n-7 | 2.00 ± 0.09 | 1.60 ± 0.07 | 2.88 ± 0.13 | ND |
| C20:1 | 1.87 ± 0.11 | 1.76 ± 0.07 | 5.81 ± 0.17 | ND |
| Total MUFA | 9.26 ± 0.52 | 11.23 ± 0.44 | 20.72 ± 0.67 | 29.89 ± 0.72 |
| C18:3n-3 | 1.66 ± 0.03 | 1.78 ± 0.04 | 1.48 ± 0.06 | 0.70 ± 0.05 |
| C20:5n-3 | 10.37 ± 0.34 | 11.87 ± 0.29 | 13.06 ± 0.64 | ND |
| C22:5n-3 | 0.93 ± 0.06 | 0.82 ± 0.06 | 0.60 ± 0.05 | ND |
| C22:6n-3 | 31.14 ± 0.67 | 24.91 ± 0.28 | 18.15 ± 0.97 | ND |
| Total n-3 PUFA | 44.10 ± 1.22 | 39.38 ± 1.11 | 33.29 ± 0.96 | 0.70 ± 0.05 |
| C18:2n-6 | 3.86 ± 0.29 | 3.98 ± 0.06 | 2.21 ± 0.09 | 53.42 ± 1.33 |
| C20:2n-6 | 0.73 ± 0.02 | 0.74 ± 0.01 | 0.52 ± 0.05 | ND |
| C20:3n-6 | 0.12 ± 0.01 | 0.23 ± 0.00 | 0.45 ± 0.04 | ND |
| C20:4n-6 | 1.12 ± 0.06 | 1.28 ± 0.02 | 2.83 ± 0.12 | ND |
| Total n-6 PUFA | 5.83 ± 0.21 | 6.23 ± 0.16 | 6.01 ± 0.21 | 53.42 ± 1.33 |
3.2. Patient Characteristics

3.3. Improvement in Disease Activity
| Variables | HMLE Group | Placebo Group | p-Value a | ||||
|---|---|---|---|---|---|---|---|
| Total (n = 18) | Men (n = 6) | Women (n = 12) | Total (n = 24) | Men (n = 6) | Women (n = 18) | ||
| Subject characteristics | |||||||
| Age (years) | 56.6 ± 2.8 | 60.5 ± 2.6 | 54.7 ± 4.0 | 58.3 ± 2.18 | 57.7 ± 4.4 | 58.4 ± 2.6 | 0.642 |
| BMI (kg/m2) | 22.4 ± 0.60 | 23.3 ± 1.2 | 22.0 ± 0.65 | 22.5 ± 0.42 | 23.0 ± 0.83 | 22.4 ± 0.50 | 0.863 |
| SBP (mmHg) | 118.9 ± 3.4 | 129.7 ± 3.7 | 113.6 ± 4.0 | 124.0 ± 2.7 | 133.2 ± 3.0 | 120.9 ± 3.1 | 0.246 |
| DBP (mmHg) | 76.6 ± 2.3 | 84.0 ± 3.7 | 72.9 ± 2.3 | 80.3 ± 1.5 | 87.3 ± 1.8 | 78.0 ± 1.5 | 0.163 |
| Duration of RA (years) | 7.1 ± 0.76 | 7.8 ± 0.63 | 6.7 ± 1.10 | 8.0 ± 0.87 | 5.4 ± 0.90 | 8.8 ± 1.1 | 0.447 |
| Total number of tender joints | 10.6 ± 0.44 | 10.5 ± 0.56 | 10.7 ± 0.60 | 9.5 ± 0.60 | 8.5 ± 0.67 | 9.8 ± 0.76 | 0.150 |
| Total number of swollen joints | 7.3 ± 0.69 | 8.3 ± 1.4 | 6.8 ± 0.74 | 7.8 ± 0.73 | 7.7 ± 1.6 | 7.8 ± 0.85 | 0.623 |
| Morning stiffness (minutes) | 69.7 ± 6.3 | 67.5 ± 13.3 | 70.8 ± 7.1 | 72.7 ± 8.0 | 66.7 ± 10.2 | 74.7 ± 10.2 | 0.782 |
| ESR (mm/h) | 50.8 ± 6.4 | 72.3 ± 9.5 | 40.0 ± 6.6 | 53.3 ± 6.1 | 47.8 ± 14.3 | 55.1 ± 6.9 | 0.785 |
| CRP (mg/dL) | 14.4 ± 1.7 | 16.0 ± 4.4 | 13.7 ± 1.5 | 16.3 ± 2.0 | 11.9 ± 3.5 | 17.8 ± 2.3 | 0.490 |
| DAS(28) | 5.80 ± 0.12 | 6.18 ± 0.09 | 5.60 ± 0.15 | 5.71 ± 0.15 | 5.47 ± 0.35 | 5.79 ± 0.16 | 0.657 |
| Physician global (0–100 mm) | 42.9 ± 2.6 | 44.0 ± 4.3 | 42.4 ± 3.3 | 40.9 ± 2.8 | 41. 7 ± 4.8 | 40.7 ± 3.4 | 0.605 |
| Patient global (0–100 mm) | 53.5 ± 2.7 | 55.0 ± 4.3 | 52.8 ± 3.6 | 47.9 ± 2.6 | 48.7 ± 4.8 | 47.6 ± 3.2 | 0.153 |
| CDAI | 27.5 ± 1.4 | 28.7 ± 2.4 | 26.9 ± 1.8 | 26.1 ± 1.6 | 25.2 ± 2.9 | 26.4 ± 2.0 | 0.534 |
| Patients taking medicines | |||||||
| Methotrexate | 15 | 5 | 10 | 19 | 8 | 11 | 0.734 |
| Corticosteroid | 9 | 4 | 5 | 13 | 5 | 8 | 0.789 |
| NSAIDS | 4 | 2 | 2 | 6 | 2 | 4 | 0.834 |
| Chinese patent medicine | 9 | 5 | 4 | 11 | 5 | 6 | 0.789 |
| TNF blockers | 2 | 0 | 2 | 2 | 1 | 1 | 0.762 |
| Variables | HMLE (18) | Placebo (24) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Month 0 | Month 6 | Difference (95% CIs) | Month 0 | Month 6 | Difference (95% Cis) | Time | Group | Time × Group | |
| TBIL (µmol/L) | 14.09 ± 1.38 | 11.98 ± 1.39 | −2.11 (−4.84, 0.62) | 13.50 ± 0.91 | 13.56 ± 1.17 | 0.054 (−3.40, 3.51) | 0.363 | 0.704 | 0.338 |
| DBIL (µmol /L) | 4.11 ± 0.42 | 3.77 ± 0.53 | −0.34 (−1.44, 0.76) | 3.83 ± 0.33 | 4.97 ± 0.50 | 1.14 (−0.018, 2.30) | 0.316 | 0.370 | 0.068 |
| IBIL (µmol /L) | 9.98 ± 1.15 | 8.21 ± 0.95 | −1.77 (−3.54, 0.03) | 9.68 ± 0.66 | 8.59 ± 0.73 | −1.09 (−3.45, 1.27) | 0.065 | 0.972 | 0.652 |
| ALT (U/L) | 26.44 ± 2.63 | 26.11 ± 5.20 | −0.33 (−9.32, 8.66) | 23.50 ± 2.37 | 27.13 ± 4.59 | 3.63 (−2.04, 9.29) | 0.502 | 0.847 | 0.420 |
| AST (U/L) | 27.22 ± 1.84 | 27.56 ± 3.41 | 0.33 (−8.20, 8.86) | 23.67 ± 3.56 | 25.00 ± 3.22 | 1.33 (−9.32, 11.99) | 0.197 | 0.890 | 0.246 |
| γ-GT (U/L) | 20.00 ± 2.37 | 16.78 ± 1.40 | −3.22 (−7.36, 0.92) | 24.58 ± 2.66 | 25.50 ± 1.93 | 0.92 (−5.24, 7.07) | 0.551 | 0.012 | 0.287 |
| TP (g/L) | 78.67 ± 1.18 | 78.79 ± 0.89 | 0.033 (−3.30, 3.37) | 78.65 ± 0.76 | 78.78 ± 0.82 | 0.12 (−1.79, 2.03 | 0.930 | 0.974 | 0.960 |
| ALB (g/L) | 48.04 ± 0.67 | 48.44 ± 0.60 | 0.39 (−1.64, 2.43) | 47.58 ± 0.39 | 45.66 ± 0.53 | −0.92 (−2.16, 0.32) | 0.629 | 0.048 | 0.231 |
| GLB (g/L) | 30.26 ± 0.68 | 30.62 ± 0.92 | −0.36 (−2.67, 1.94) | 31.07 ± 0.69 | 32.11 ± 0.57 | 1.04 (−0.66, 2.74) | 0.641 | 0.138 | 0.302 |
| BUN (mmol/L) | 5.58 ± 0.25 | 5.30 ± 0.26 | −0.28 (−1.06, 0.49) | 5.23 ± 0.31 | 5.34 ± 0.37 | 0.11 (−0.63, 0.85) | 0.745 | 0.667 | 0.457 |
| Cr (mmol/L) | 64.21 ± 3.30 | 68.55 ± 3.33 | 4.34 (−5.49, 14.17) | 66.61 ± 2.68 | 63.08 ± 3.39 | −3.53 (−10.72, 3.65) | 0.887 | 0.668 | 0.174 |
| UA (mmol/L) | 319.8 ± 22.3 | 280.5 ± 17.3 | −39.3 (−97.9, 19.4) | 296.4 ± 16.1 | 294.1 ± 18.9 | −2.2 (−49.3, 44.9) | 0.305 | 0.807 | 0.251 |
| Adverse Events | HMLE Group (18) | Placebo Group (24) | p-Value a |
|---|---|---|---|
| Elevated ALT | 2 | 3 | 0.891 |
| Elevated AST | 1 | 2 | 0.729 |
| Loss of appetite | 8 | 12 | 0.721 |
| Nausea | 5 | 7 | 0.921 |
| Vomiting | 4 | 5 | 0.914 |
| Variables | HMLE Group (n = 18) | Placebo Group (n = 24) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | Time | Group | Time × Group | |
| Total energy (kcal) | 1858.9 ± 40.6 | 1843.8 ± 41.6 | 1846.5 ± 44.5 | 1824.2 ± 38.6 | 0.072 | 0.788 | 0.726 |
| Carbohydrate (g) | 261.0 ± 8.4 | 259.4 ± 7.6 | 269.5 ± 9.0 | 265.3 ± 8.6 | 0.199 | 0.557 | 0.558 |
| Total fat (g) | 52.6 ± 1.0 | 54.1 ± 1.4 * | 49.9 ± 1.2 | 51.3 ± 1.2 * | 0.003 | 0.117 | 0.832 |
| Protein (g) | 85.0 ± 4.1 | 79.7 ± 6.0 | 79.1 ± 4.6 | 75.3 ± 4.1 | 0.061 | 0.42 | 0.759 |
| Carbohydrate (% of energy) | 55.9 ± 0.88 | 56.1 ± 0.10 | 58.1 ± 0.78 | 57.9 ± 0.97 | 0.956 | 0.126 | 0.584 |
| Fat (% of energy) | 25.8 ± 0.78 | 26.7 ± 0.92 * | 24.9 ± 0.80 | 25.6 ± 0.75 * | 0.001 | 0.358 | 0.705 |
| Protein (% of energy) | 18.3 ± 0.66 | 17.1 ± 1.0 | 17.1 ± 0.87 | 16.6 ± 0.85 | 0.073 | 0.445 | 0.512 |
3.4. Effect of HMLE Supplementation on Blood Levels of Fatty Acids Composition and Inflammatory Mediators and Cytokines


| Variables | HMLE Group (n = 18) | Placebo Group (n = 24) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Month 0 | Month 3 | Month 6 | Month 0 | Month 3 | Month 6 | Time | Group | Time × Group | |
| SBP (mmHg) | 118.9 ± 3.4 | 118.2 ± 2.2 | 121.4 ± 2.8 * | 124.0 ± 2.7 | 124.4 ± 2.0 | 122.8 ± 2.4 | 0.610 | 0.238 | 0.056 |
| DBP (mmHg) | 76.6 ± 2.3 | 75.7 ± 1.5 | 78.4 ± 1.9 | 80.3 ± 1.5 | 81.0 ± 1.4 | 78.7 ± 1.1 | 0.944 | 0.143 | 0.007 |
| TJC (28) | 10.6 ± 0.4 | 8.7 ± 0.5 ** | 5.1 ± 0.5 ** | 9.5 ± 0.6 | 8.2 ± 0.7 ** | 6.9 ± 0.5 ** | <0.01 | 0.917 | <0.01 |
| SJC (28) | 7.3 ± 0.7 | 5.9 ± 0.5 ** | 4.1 ± 0.5 ** | 7.8 ± 0.73 | 6.5 ± 0.7 ** | 5.3 ± 0.6 ** | <0.01 | 0.417 | 0.053 |
| Morning stiffness (min) | 69.7 ± 6.3 | 51.1 ± 7.7 ** | 40.6 ± 7.0 ** | 72.7 ± 8.0 | 62.9 ± 7.6 ** | 58.1 ± 6.6 ** | <0.01 | 0.296 | 0.016 |
| ESR | 50.8 ± 6.4 | 48.0 ± 5.3 | 34.7 ± 4.7 ** | 53.3 ± 6.1 | 49.2 ± 4.8 | 38.9 ± 3.9 ** | <0.01 | 0.719 | 0.571 |
| CRP | 14.4 ± 1.7 | 12.0 ± 1.0 * | 11.4 ± 1.5 ** | 16.3 ± 2.0 | 15.1 ± 1.8 | 14.9 ± 1.6 * | <0.01 | 0.236 | 0.273 |
| RF | 204.6 ± 33.1 | 196.1 ± 24.6 | 183.7 ± 23.8 | 211.7 ± 23.0 | 204.1 ± 22.8 | 201.6 ± 20.9 | 0.057 | 0.743 | 0.650 |
| DAS(28) | 5.80 ± 0.12 | 5.51 ± 0.11 ** | 4.69 ± 0.12 ** | 5.71 ± 0.15 | 5.46 ± 0.14 ** | 5.07 ± 0.14 ** | <0.01 | 0.678 | <0.01 |
| Physician global | 42.9 ± 2.6 | 40.4 ± 2.3 * | 37.2 ± 2.2 ** | 40.9 ± 2.8 | 39.4 ± 2.5 | 36.4 ± 2.1 ** | <0.01 | 0.707 | 0.753 |
| Patient global | 53.5 ± 2.7 | 47.7 ± 2.2 ** | 42.4 ± 2.3 ** | 47.9 ± 2.6 | 44.8 ± 2.2 * | 40.6 ± 2.1 ** | <0.01 | 0.288 | 0.135 |
| CDAI | 27.5 ± 1.4 | 23.4 ± 1.2 ** | 17.2 ± 1.3 ** | 26.1 ± 1.6 | 23.1 ± 1.6 ** | 19.9 ± 1.4 ** | <0.01 | 0.861 | <0.01 |
| Inflammatory factors | |||||||||
| TNF-α | 71.3 ± 7.4 | 53.9 ± 6.6 ** | 39.8 ± 5.0 ** | 81.5 ± 5.5 | 67.1 ± 5.1 ** | 59.5 ± 4.7 ** | <0.01 | 0.076 | 0.029 |
| IL-1β | 127.8 ± 10.8 | 108.9 ± 3.0 * | 97.1 ± 3.3 ** | 138.7 ± 10.0 | 115.4 ± 8.8 ** | 107.5 ± 6.1 ** | <0.01 | 0.377 | 0.739 |
| IL-6 | 109.4 ± 5.8 | 109.9 ± 4.6 | 106.4 ± 4.1 | 96.7 ± 8.8 | 95.4 ± 9.0 | 94.4 ± 9.1 | 0.130 | 0.245 | 0.617 |
| IL-10 | 54.7 ± 5.2 | 68.1 ± 5.8 ** | 78.4 ± 5.6 ** | 48.1 ± 3.8 | 59.1 ± 3.8 ** | 65.1 ± 3.6 ** | <0.01 | 0.138 | 0.014 |
| PGE2 | 438.2 ± 17.7 | 405.2 ± 20.6 ** | 379.5 ± 21.2 ** | 429.3 ± 15.5 | 402.6 ± 14.5 ** | 389.9 ± 14.4 ** | <0.01 | 0.988 | <0.01 |
| HMLE Group (n = 18) | Placebo Group (n = 24) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M0 | M6 | Difference (95% CIs) | M0 | M6 | Difference (95% CIs) | Time | Group | Time × Group | |
| C14:0 | 0.12 | 0.14 | 0.017 (−0.010,0.045) | 0.10 | 0.13 | 0.026 (−0.039, 0.091) | 0.101 | 0.155 | 0.745 |
| C15:0 | 0.06 | 0.08 | 0.019 (0.007, 0.033) ** | 0.06 | 0.06 | 0.001 (−0.014, 0.016) | 0.067 | 0.053 | 0.095 |
| C16:0 | 21.40 | 22.97 | 1.57 (−0.30, 3.44) | 21.03 | 23.15 | 2.12 (−0.73, 4.96) | 0.032 | 0.909 | 0.734 |
| C17:0 | 0.22 | 0.28 | 0.064 (0.031, 0.097) ** | 0.19 | 0.22 | 0.029 (−0.024, 0.082) | <0.01 | 0.018 | 0.228 |
| C18:0 | 15.35 | 14.93 | −0.42 (−1.79,0.95) | 14.72 | 14.78 | 0.062 (−4.77, 4.89) | 0.808 | 0.514 | 0.745 |
| C20:0 | 0.29 | 0.37 | 0.081 (−0.039, 0.20) | 0.25 | 0.45 | 0.21 (−0.060, 0.47) | 0.018 | 0.700 | 0.276 |
| C22:0 | 0.62 | 0.63 | 0.012 (−0.11, 0.13) | 0.63 | 0.66 | 0.035 (−0.21, 0.28) | 0.663 | 0.851 | 0.833 |
| C24:0 | 1.69 | 1.70 | 0.014 (−0.47, 0.50) | 1.80 | 1.29 | −0.51 (−1.05, 0.076) | 0.186 | 0.507 | 0.165 |
| SFA | 39.76 | 41.12 | 1.36 (−2.06, 4.77) | 38.79 | 40.72 | 1.93 (−3.47, 7.30) | 0.277 | 0.652 | 0.851 |
| C16:1n-7 | 0.12 | 0.14 | 0.019 (−0.018, 0.056) | 0.12 | 0.15 | 0.027 (−0.027, 0.081) | 0.162 | 0.998 | 0.791 |
| C18:1n-9 | 14.23 | 13.62 | −0.60 (−1.81, 0.61) | 13.90 | 15.67 | 1.77 (−1.25, 4.79) | 0.637 | 0.502 | 0.344 |
| C20:1n-9 | 0.49 | 0.36 | −0.14 (−0.22, −0.058) ** | 0.48 | 0.45 | −0.028 (−0.36, 0.30) | 0.089 | 0.458 | 0.249 |
| C22:1n-9 | 0.38 | 0.20 | −0.170 (−0.36, −0.01) * | 0.28 | 0.30 | 0.016 (−0.45, 0.48) | 0.349 | 0.982 | 0.267 |
| C24:1n-9 | 4.63 | 4.18 | −0.45 (−1.31, 0.42) | 4.53 | 3.81 | −0.72 (−2.47, 1.03) | 0.147 | 0.625 | 0.728 |
| MUFA | 19.86 | 18.51 | −1.35 (−3.06, 0.37) | 19.31 | 20.38 | 1.07 (−3.39, 5.52) | 0.912 | 0.669 | 0.352 |
| C18:3n-3 | 0.15 | 0.16 | 0.0027 (−0.028, 0.033) | 0.16 | 0.13 | −0.032 (−0.11, 0.044) | 0.321 | 0.784 | 0.242 |
| C20:3n-3 | 0.11 | 0.08 | −0.038 (−0.071, −0.005) * | 0.10 | 0.06 | −0.038 (−0.14, 0.062) | 0.035 | 0.414 | 0.993 |
| C20:5n-3 | 0.59 | 1.36 | 0.77 (0.39, 1.16) ** | 0.45 | 0.59 | 0.14 (−0.18, 0.46) | <0.01 | 0.017 | 0.057 |
| C22:5n-3 | 2.01 | 2.25 | 0.24 (−0.18, 0.67) | 2.22 | 1.36 | −0.86 (−1.57, −0.16) * | 0.107 | <0.01 | 0.214 |
| C22:6n-3 | 4.29 | 5.23 | 0.94 (0.041, 2.27) * | 4.38 | 3.58 | −0.80 (−2.9, 1.29) | 0.891 | 0.160 | 0.017 |
| n-3 PUFA | 7.14 | 9.07 | 1.92 (0.091, 3.76) * | 7.31 | 5.73 | −1.59 (−4.26, 1.08) | 0.831 | 0.101 | 0.036 |
| C18:2n-6 | 12.65 | 13.79 | 1.13 (−0.65, 2.92) | 12.34 | 16.45 | 4.11 (0.77, 7.59) ** | <0.01 | 0.196 | 0.102 |
| C18:3n-6 | 0.03 | 0.02 | −0.0063 (−0.029, 0.017) | 0.02 | 0.03 | 0.011 (−0.023, 0.045) | 0.804 | 0.858 | 0.380 |
| C20:2n-6 | 0.34 | 0.29 | −0.041 (−0.097, 0.015) | 0.31 | 0.32 | 0.0077 (−0.18, 0.19) | 0.573 | 0.919 | 0.411 |
| C20:3n-6 | 1.42 | 1.30 | −0.12 (−0.43, 0.20) | 1.73 | 1.55 | −0.18 (−1.12, 0.75) | 0.352 | 0.055 | 0.835 |
| C20:4n-6 | 16.07 | 14.02 | −2.05 (−4.19, 0.095) | 17.02 | 13.12 | −3.90 (−6.79, 1.99) | 0.015 | 0.982 | 0.410 |
| C22:2n-6 | 0.10 | 0.09 | −0.0088 (−0.075, 0.057) | 0.04 | 0.09 | 0.051 (−0.11, 0.21) | 0.495 | 0.338 | 0.569 |
| C22:4n-6 | 2.64 | 1.79 | −0.85 (−1.17, −0.53) ** | 3.13 | 1.64 | −1.49 (−2.46, −0.51) * | <0.01 | 0.489 | 0.065 |
| n-6 PUFA | 33.23 | 31.30 | −1.93 (−5.62, 1.76) | 34.59 | 33.20 | −1.39 (−4.64, 1.86) | 0.289 | 0.350 | 0.860 |

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burmester, G.R.; Rubbert-Roth, A.; Cantagrel, A.; Hall, S.; Leszczynski, P.; Feldman, D.; Rangaraj, M.J.; Roane, G.; Ludivico, C.; Lu, P.; et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann. Rheum. Dis. 2014, 73, 69–74. [Google Scholar] [CrossRef]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Padyukov, L.; Seielstad, M.; Ong, R.T.; Ding, B.; Rönnelid, J.; Seddighzadeh, M.; Alfredsson, L.; Klareskog, L. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 259–265. [Google Scholar] [CrossRef]
- Smith, H.S.; Smith, A.R.; Seidner, P. Painful rheumatoid arthritis. Pain. Physician. 2011, 14, E427–E458. [Google Scholar] [PubMed]
- Klareskog, L.; Catrina, A.I.; Paget, S. Rheumatoid arthritis. Lancet 2009, 373, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Walker-Bone, K. Natural remedies in the treatment of osteoarthritis. Drugs. Aging. 2003, 20, 517–526. [Google Scholar] [CrossRef]
- Brennan, F.M.; McInnes, I.B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Investig. 2008, 118, 3537–3545. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Grabowska, A.; Chain, B. Nitric oxide up-regulates the release of inflammatory mediators by mouse macrophages. Eur. J. Immunol. 1995, 25, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.Y.; Freire-Moar, J.; Sigal, E.; Chu, C.Q. Expression of cyclooxygenase-2 in human and an animal model of rheumatoid arthritis. Br. J. Rheumatol. 1996, 35, 711–718. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.M.; Katschke, K.J.; Volin, M.V.; Ruth, J.H.; Woodruff, D.C.; Amin, M.A.; Connors, M.A.; Kurata, H.; Arai, K.; Haines, G.K.; et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J. Immunol. 2001, 166, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.M. Interleukin 10 and arthritis. Rheumatology (Oxf.) 1999, 38, 293–297. [Google Scholar] [CrossRef]
- Li, G.P.; Fu, Y.Q.; Zheng, J.S.; Li, D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs 2014, 12, 568–588. [Google Scholar] [CrossRef] [PubMed]
- Li, G.P.; Li, J.; Li, D. Seasonal variation in nutrient composition of Mytilus coruscus from china. J. Agric. Food Chem. 2010, 58, 7831–7837. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sinclair, A.; Mann, N.; Turner, A.; Ball, M.; Kelly, F.; Abedin, L.; Wilson, A. The association of diet and thrombotic risk factors in healthy male vegetarians and meat-eaters. Eur. J. Clin. Nutr. 1999, 53, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Fransen, J.; van Riel, P.L. The Disease Activity Score and the EULAR response criteria. Clin. Exp. Rheumatol. 2005, 23, S93–S99. [Google Scholar] [PubMed]
- Smolen, J.S.; Breedveld, F.C.; Schiff, M.H.; Kalden, J.R.; Emery, P.; Eberl, G.; van Riel, P.L.; Tugwell, P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxf.) 2003, 42, 244–257. [Google Scholar] [CrossRef]
- Reed, G.W.; Leung, K.; Rossetti, R.G.; Vanbuskirk, S.; Sharp, J.T.; Zurier, R.B. Treatmeng of rheumatoid arthritis with marine and botanical oils: An 18-month, randomized, and double-blind trial. Evid Based Complement Altern. Med. 2014, 2014, 857456. [Google Scholar] [CrossRef]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.G.; Gibson, S.L.; Conway, V.; Chappell, D. Perna canaliculus in the treatment of arthritis. Practitioner 1980, 224, 955–960. [Google Scholar] [PubMed]
- Gibson, S.L.M.; Gibson, R.G. The treatment of arthritis with a lipid extract of Perna Canaliculus: A randomized trial. Complement. Ther. Med. 1998, 6, 122–126. [Google Scholar] [CrossRef]
- Lau, C.S.; Chiu, P.K.Y.; Chu, E.M.Y.; Cheng, I.Y.W.; Tang, W.M.; Man, R.Y.K.; Halpern, G.M. Treatment of knee osteoarthritis with Lyprinol, lipid extract of the green-lipped mussel- a double-blind placebo-controlled study. Progr. Nutr. 2004, 6, 17–31. [Google Scholar]
- Nodari, S.; Triggiani, M.; Campia, U.; Manerba, A.; Milesi, G.; Cesana, B.M.; Gheorghiade, M.; Dei Cas, L. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M. N-3 fatty acid supplements in rheumatoid arthritis. Am. J. Clin. Nutr. 2000, 71, 349S–351S. [Google Scholar] [PubMed]
- Lu, L.J.; Bao, C.D.; Dai, M.; Teng, J.L.; Fan, W.; Du, F.; Yang, N.P.; Zhao, Y.H.; Chen, Z.W.; Xu, J.H.; et al. Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum. 2009, 61, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Singla, M.; Gupta, N.; Goyal, P.; Sagar, V.; Sharma, A.; Khanna, S.; Singh, S. Randomized controlled trial comparing 2 different starting doses of methotrexate in rheumatoid arthritis. Clin. Ther. 2014, 36, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.G.; Gibson, S.L.M. Green-lipped mussel extract in arthritis. Lancet 1981, 1, 439. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G.M. Anti-inflammatory effects of a stabilized lipid extract of Perna canaliculus (Lyprinol). Allerg. Immunol. 2000, 32, 272–278. [Google Scholar]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Rheumatoid arthritis. Cell 1996, 85, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996, 14, 397–440. [Google Scholar] [CrossRef] [PubMed]
- Keffer, J.; Probert, L.; Cazlaris, H.; Georgopoulos, S.; Kaslaris, E.; Kioussis, D.; Kollias, G. Transgenic mice expressing human tumor necrosis factor: A predictive genetic model of arthritis. EMBO J. 1991, 10, 4025–4031. [Google Scholar] [PubMed]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerim in osteoblastic stromal cell. Endocrinology 1998, 139, 4743–4746. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Manning, C.; Tsay, A.; Naito, A.; Pan, C.; Amento, E.; Goldring, S.R. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000, 43, 250–258. [Google Scholar]
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Shigeyama, Y.; Pap, T.; Kunzler, P.; Simmen, B.R.; Gay, R.E.; Gay, S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Maini, R.N.; Taylor, P.C. Anti-cytoline therapy for rheumatoid arthritis. Annu. Rev. Med. 2000, 51, 207–229. [Google Scholar] [CrossRef]
- Miossec, P. Interleukin-17 in rheumatoid arthritis: If T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003, 48, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.; Netea, M.G.; Kim, S.H.; Yoon, D.Y.; Oppers-Walgreen, B.; Radstake, T.R.; Barrera, P.; van de Loo, F.A.; Dinarello, C.A.; van den Berg, W.B. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2006, 103, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Wu, H. In vivo evidence for prostaglandin inhibitory activity in New Zealand green-lipped mussel extract. N. Z. Med. J. 1984, 97, 355–357. [Google Scholar] [PubMed]
- Whitehouse, M.W.; Macrides, T.A.; Kalafatis, N.; Betts, W.H. Anti-inflammatory activity of a lipid fraction (lyprinol) from the NZ green-lipped mussel. Inflammopharmacology 1997, 5, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Murphy, K.J.; Li, D. Marine lipids: Overeview “news insights and lipid composition of Lyprinol”. Allerg. Immunol. 2000, 32, 261–271. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Li, G.; Zhang, X.; Xing, G.; Hu, X.; Yang, L.; Li, D. Lipid Extract from Hard-Shelled Mussel (Mytilus coruscus) Improves Clinical Conditions of Patients with Rheumatoid Arthritis: A Randomized Controlled Trial. Nutrients 2015, 7, 625-645. https://doi.org/10.3390/nu7010625
Fu Y, Li G, Zhang X, Xing G, Hu X, Yang L, Li D. Lipid Extract from Hard-Shelled Mussel (Mytilus coruscus) Improves Clinical Conditions of Patients with Rheumatoid Arthritis: A Randomized Controlled Trial. Nutrients. 2015; 7(1):625-645. https://doi.org/10.3390/nu7010625
Chicago/Turabian StyleFu, Yuanqing, Guipu Li, Xinhua Zhang, Gengyan Xing, Xiaojie Hu, Lifeng Yang, and Duo Li. 2015. "Lipid Extract from Hard-Shelled Mussel (Mytilus coruscus) Improves Clinical Conditions of Patients with Rheumatoid Arthritis: A Randomized Controlled Trial" Nutrients 7, no. 1: 625-645. https://doi.org/10.3390/nu7010625
APA StyleFu, Y., Li, G., Zhang, X., Xing, G., Hu, X., Yang, L., & Li, D. (2015). Lipid Extract from Hard-Shelled Mussel (Mytilus coruscus) Improves Clinical Conditions of Patients with Rheumatoid Arthritis: A Randomized Controlled Trial. Nutrients, 7(1), 625-645. https://doi.org/10.3390/nu7010625