Ginseng Berry Extract Supplementation Improves Age-Related Decline of Insulin Signaling in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Korean GB Extract
2.3. Cell Culture
2.4. Animals
2.5. Glucose Tolerance Tests
2.6. Insulin Tolerance Tests
2.7. Measurement of Blood Glucose, Serum Insulin and Serum Lipid Level
2.8. Measurement of Fat Mass
2.9. HOMA-IR Calculation
2.10. Immunohistochemical and Histological Staining of Pancreatic Sections
2.11. Western Blotting and Immunoprecipitation
2.12. Quantitative Real-Time-PCR (qRT-PCR) Analysis
2.13. Statistical Analyses
3. Results
3.1. GB Extract Supplementation Did not Affect the Body Weight, Blood Glucose Levels or Serum Lipid Levels in Aged Mice

3.2. GB Extract Supplementation Increased Insulin Sensitivity in Aged Mice
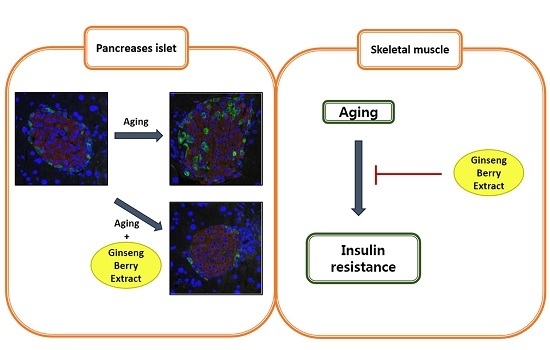
3.3. GB Extract Supplementation Ameliorated Pancreatic Islet Hypertrophy in Aged Mice and Decreased Serum Insulin Levels



3.4. GB Extract Supplementation Increased Phosphorylation of IRS-1 and AKT in Muscle of Aged Mice
3.5. GB Extract Supplementation Increased Phosphorylation of IRS-1 and AKT in C2C12 Cells


3.6. GB Extract Supplementation Increased the Expression of FOXO1 and PPARγ mRNA and Protein

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Picard, M. Pathways to aging: The mitochondrion at the intersection of biological and psychosocial sciences. J. Aging Res. 2011, 2011, 814096. [Google Scholar] [CrossRef] [PubMed]
- De Tata, V. Age-related impairment of pancreatic beta-cell function: Pathophysiological and cellular mechanisms. Front. Endocrinol. 2014, 5, 138. [Google Scholar]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. The metabolic syndrome-from insulin resistance to obesity and diabetes. Med. Clin. N. Am. 2011, 95, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Brissova, M.; Reinert, R.B.; Nyman, L.; Liu, E.H.; Thompson, C.; Shostak, A.; Shiota, M.; Takahashi, T.; Powers, A.C. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 2013, 62, 4144–4153. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Xia, X.; Mou, T.; Jiang, B.; Fan, D.; Wang, P.; Liu, Y.; Hou, Y.; Zhao, Y. Anti-tumor activity of three ginsenoside derivatives in lung cancer is associated with wnt/beta-catenin signaling inhibition. Eur. J. Pharmacol. 2014. [Google Scholar] [CrossRef]
- Jang, H.J.; Han, I.H.; Kim, Y.J.; Yamabe, N.; Lee, D.; Hwang, G.S.; Oh, M.; Choi, K.C.; Kim, S.N.; Ham, J.; et al. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J. Agric. Food Chem. 2014, 62, 2830–2836. [Google Scholar] [CrossRef]
- Ramesh, T.; Kim, S.W.; Sung, J.H.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Effect of fermented panax ginseng extract (ginst) on oxidative stress and antioxidant activities in major organs of aged rats. Exp. Gerontol. 2012, 47, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, J.W.; Jo, J.Y.; Hwang, D.S.; Lee, C.H.; Jang, J.B.; Lee, K.S.; Yeo, I.; Lee, J.M. Effect of korean red ginseng on cold hypersensitivity in the hands and feet: Study protocol for a randomized controlled trial. Trials 2013, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Korean red ginseng for allergic rhinitis. Immunopharmacol. Immunotoxicol. 2013, 35, 693. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Waki, I.; Chujo, T.; Kikuchi, T.; Hiyama, C.; Yamazaki, K.; Tanaka, O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J. Pharmacobio-Dyn. 1981, 4, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Mehendale, S.R.; Wang, A.; Han, A.H.; Wu, J.A.; Osinski, J.; Yuan, C.S. American ginseng leaf: Ginsenoside analysis and hypoglycemic activity. Pharmacol. Res. 2004, 49, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; McHendale, S.; Yuan, C.S. Ginseng and diabetes. Am. J. Chin. Med. 2005, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Dey, L.; Xie, J.T.; Wang, A.; Wu, J.; Maleckar, S.A.; Yuan, C.S. Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine 2003, 10, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, H.J.; Kim, Y.K.; Park, S.U.; Choi, J.E.; Cha, J.Y.; Jun, H.S. Increase in insulin secretion induced by panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocininduced diabetic mice. J. Ginseng Res. 2012, 36, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Wojtaszewski, J.F. Role of akt substrate of 160 kda in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metab. 2007, 32, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tanti, J.F.; Gual, P.; Gremeaux, T.; Gonzalez, T.; Barres, R.; Le Marchand-Brustel, Y. Alteration in insulin action: Role of irs-1 serine phosphorylation in the retroregulation of insulin signalling. Ann. D’endocrinol. 2004, 65, 43–48. [Google Scholar] [CrossRef]
- Chen, Q.; Ames, B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast f65 cells. Proc. Natl. Acad. Sci. USA 1994, 91, 4130–4134. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Daitoku, H.; Hatta, M.; Tanaka, K.; Fukamizu, A. Insulin-induced phosphorylation of fkhr (foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. USA 2003, 100, 11285–11290. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Ignacimuthu, S.; Paulraj, M.G.; Vishal, R. Insulin sensitization via partial agonism of ppargamma and glucose uptake through translocation and activation of glut4 in pi3k/p-akt signaling pathway by embelin in type 2 diabetic rats. Biochim. Biophys. Acta 2013, 1830, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Lee, E.K.; Choi, Y.J.; Kim, J.M.; Kim, D.H.; Zou, Y.; Kim, C.H.; Lee, J.; Kim, H.S.; Kim, N.D.; et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J. Dent. Res. 2011, 90, 830–840. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. Nf-kappab signaling in the aging process. J. Clin. Immunol. 2009, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kowal, P.; Chatterji, S.; Naidoo, N.; Biritwum, R.; Fan, W.; Lopez Ridaura, R.; Maximova, T.; Arokiasamy, P.; Phaswana-Mafuya, N.; Williams, S.; et al. Data resource profile: The world health organization study on global ageing and adult health (sage). Int. J. Epidemiol. 2012, 41, 1639–1649. [Google Scholar] [CrossRef]
- Michalakis, K.; Goulis, D.G.; Vazaiou, A.; Mintziori, G.; Polymeris, A.; Abrahamian-Michalakis, A. Obesity in the ageing man. Metabolism 2013, 62, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.M.; Andres, A.; Ros, M.; Bogonez, E.; Arribas, C.; Fernandez-Agullo, T.; de Solis, A.J.; Gallardo, N.; Martinez, C. Development of insulin resistance during aging: Involvement of central processes and role of adipokines. Curr. Protein Pept. Sci. 2011, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.I.; Kolterman, O.G.; Griffin, J.; Olefsky, J.M. Mechanisms of insulin resistance in aging. J. Clin. Investig. 1983, 71, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.W.; Minaker, K.L.; Pallotta, J.A.; Flier, J.S. Characterization of the insulin resistance of aging. J. Clin. Investig. 1983, 71, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Borai, A.; Livingstone, C.; Kaddam, I.; Ferns, G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med. Res. Methodol. 2011, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in wistar and sprague-dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Jo, E.K.; Lee, M.S. Role of pancreatic beta-cell death and inflammation in diabetes. Diabetes Obes. Metab. 2013, 15, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mizrachi, E.; Wen, W.; Stahlhut, S.; Welling, C.M.; Permutt, M.A. Islet beta cell expression of constitutively active akt1/pkb alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Investig. 2001, 108, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, V.; Nikolov, B. Changes in pancreatic islets in aging wistar and zucker rats: A histochemical and ultrastructural morphometric study. Mech Ageing Dev. 1994, 74, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dillberger, J.E. Age-related pancreatic islet changes in sprague-dawley rats. Toxicol. Pathol. 1994, 22, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gao, N.; Gorski, R.K.; White, P.; Hardy, O.T.; Rafiq, K.; Brestelli, J.E.; Chen, G.; Stoeckert, C.J., Jr.; Kaestner, K.H. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on hnf-4alpha. Genes. Dev. 2007, 21, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Intern. Med. 2010, 25, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Brenelli, S.L.; Silva, A.C.; Nunes, A.L.; Velloso, L.A.; Saad, M.J. Effect of aging on insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of rats. Endocrinology 1996, 137, 151–159. [Google Scholar] [PubMed]
- Schenk, S.; Horowitz, J.F. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J. Clin. Investig. 2007, 117, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Leavens, K.F.; Birnbaum, M.J. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S. Oxidative stress hypothesis of aging. Free Radic. Biol. Med. 2002, 33, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P. Aging and oxidative stress: Modulation by dietary restriction. Free Radic. Biol. Med. 1996, 21, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of foxos and p53 by sirt1 modulators under oxidative stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Park, S.; Yu, B.P.; Chung, H.Y. Modulation of ppar in aging, inflammation, and calorie restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Seo, A.Y.; Chung, S.W.; Kim, M.K.; Leeuwenburgh, C.; Yu, B.P.; Chung, H.Y. Molecular mechanism of ppar in the regulation of age-related inflammation. Ageing Res. Rev. 2008, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.W.; Chen, L.; Fisher, S.J.; Szanto, I.; Ristow, M.; Jozsi, A.C.; Hirshman, M.F.; Rosen, E.D.; Goodyear, L.J.; Gonzalez, F.J.; et al. Muscle-specific ppargamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Investig. 2003, 112, 608–618. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, E.; Kim, S.; Lee, S.J.; Oh, B.-C.; Jun, H.-S. Ginseng Berry Extract Supplementation Improves Age-Related Decline of Insulin Signaling in Mice. Nutrients 2015, 7, 3038-3053. https://doi.org/10.3390/nu7043038
Seo E, Kim S, Lee SJ, Oh B-C, Jun H-S. Ginseng Berry Extract Supplementation Improves Age-Related Decline of Insulin Signaling in Mice. Nutrients. 2015; 7(4):3038-3053. https://doi.org/10.3390/nu7043038
Chicago/Turabian StyleSeo, Eunhui, Sunmi Kim, Sang Jun Lee, Byung-Chul Oh, and Hee-Sook Jun. 2015. "Ginseng Berry Extract Supplementation Improves Age-Related Decline of Insulin Signaling in Mice" Nutrients 7, no. 4: 3038-3053. https://doi.org/10.3390/nu7043038
APA StyleSeo, E., Kim, S., Lee, S. J., Oh, B. -C., & Jun, H. -S. (2015). Ginseng Berry Extract Supplementation Improves Age-Related Decline of Insulin Signaling in Mice. Nutrients, 7(4), 3038-3053. https://doi.org/10.3390/nu7043038