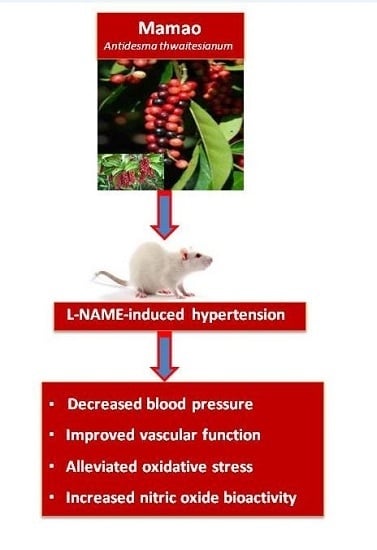
Mamao Pomace Extract Alleviates Hypertension and Oxidative Stress in Nitric Oxide Deficient Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material and Sample Preparation
2.2. Antioxidant Assays
2.3. In Vivo Assessment for the Effect of MP
2.3.1. Hemodynamic Measurements
2.3.2. Oxidative Stress Biomarkers
2.3.3. Western Blot Analysis
2.4. Statistical Analysis
3. Results
3.1. Antioxidant Activity of MP
| Assay | Ascorbic Acid | MP |
|---|---|---|
| DPPH (IC50) | 5.03 ± 0.21 (µg/mL) * | 600 ± 10 (µg/mL) |
| FRAP | 10.1 ± 0.9 (mg ascorbate/g MP) | |
| 114.7 ± 10.2 (meq /kg MP) | ||
| Total anthocyanin content | 4.87 ± 0.03 (mg cyanindin-3-glucoside/g MP) |
3.2. Effect of MP on Hemodynamics in l-NAME-Induced Hypertension Rats

| Parameters | Normal Control | L-NAME | ||||
|---|---|---|---|---|---|---|
| Vehicle | MP100 (mg/kg) | MP300 (mg/kg) | Vehicle | MP100 (mg/kg) | MP300 (mg/kg) | |
| SBP (mmHg) | 118 ± 3 | 128 ± 4 | 127 ± 4 | 192 ± 4 * | 158 ± 4 *,# | 141 ± 3 *,#,† |
| DBP (mmHg) | 76 ± 3 | 77 ± 2 | 82 ± 3 | 134 ± 4 * | 108 ± 3 *,# | 90 ± 2 *,#,† |
| MAP (mmHg) | 93 ± 3 | 96 ± 2 | 100 ± 5 | 156 ± 4 * | 129 ± 2 *,# | 112 ± 2 *,#,† |
| HR (beats/min) | 368 ± 43 | 352 ± 21 | 383 ± 5 | 348 ± 26 | 386 ± 18 | 387 ± 13 |
| HBF (mL/min/100 g tissue) | 8.2 ± 0.5 | 8.3 ± 0.3 | 8.2 ± 0.5 | 4.2 ± 0.5 * | 6.2 ± 0.4 *,# | 7.3 ± 0.3 ,#,† |
| HVR (mmHg/mL/min/100 g tissue) | 11.7 ± 0.9 | 11.9 ± 0.6 | 11.6 ± 0.8 | 44.3 ±5.7 * | 21.0 ±1.9 *,# | 12.4 ± 0.4 ,#,† |
3.3. Effect of MP on Vascular Reactivity
3.4. Effect of MP on Oxidative Stress in L-NAME-Treated Rats



3.5. Effect of MP on l-NAME-Induced Suppression of Nitric Oxide Formation

3.6. Effect of MP on eNOS Protein Expression

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lin, J.K.; Singh, G.M.; Paciorek, C.J.; Cowan, M.J.; Farzadfar, F.; Stevens, G.A.; Lim, S.S.; Riley, L.M.; et al. National, regional, and global trends in systolic blood pressure since 1980: Systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 2011, 377, 568–577. [Google Scholar] [CrossRef]
- Rodrigo, R.; Gonzalez, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Schiffrin, E.L. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem. Cell Biol. 2004, 122, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Treatment of hypertension with nutraceuticals, vitamins, antioxidants and minerals. Expert Rev. Cardiovasc. Ther. 2007, 5, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Stuckless, J.; Richardson, V. Role of the immune system in hypertension: Modulation by dietary antioxidants. Int. J. Angiol. 2011, 20, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P. The Genus Antidesma (Euphorbiaceae) in Madagascar and the Comoro Islands. Kew. Bull. 1999, 54, 877–885. [Google Scholar] [CrossRef]
- Puangpronpitag, D.; Areejitranusorn, P.; Boonsiri, P.; Suttajit, M.; Yongvanit, P. Antioxidant activities of polyphenolic compounds isolated from Antidesma thwaitesianum Mull. Arg. seeds and marcs. J. Food Sci. 2008, 73, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Kropat, C.; Mueller, D.; Boettler, U.; Zimmermann, K.; Heiss, E.H.; Dirsch, V.M.; Rogoll, D.; Melcher, R.; Richling, E.; Marko, D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol. Nutr. Food Res. 2013, 57, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Basili, S.; Paoletti, V.; Davi, G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Luangaram, S.; Leekhaosoong, K.; Kukongviriyapan, V.; Preeprame, S. Antioxidant and vascular protective activities of Cratoxylum formosum, Syzygium gratum and Limnophila aromatica. Biol. Pharm. Bull. 2007, 30, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with l-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Donpunha, W.; Prachaney, P.; Phisalaphong, C. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens. Res. 2012, 35, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, V.; Sachdeva, J.; Golechha, M.; Kumari, S.; Arya, D.S. Curcumin protects rat myocardium against isoproterenol-induced ischemic injury: Attenuation of ventricular dysfunction through increased expression of Hsp27 along with strengthening antioxidant defense system. J. Cardiovasc. Pharmacol. 2010, 55, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Mezadri, T.; Villano, D.; Ferna´ndez-Pachon, M.S.; Garcıa-Parrilla, M.C.; Troncoso, A.M. Antioxidant compounds and antioxidant activity in acerola (Malpighia emarginata DC.) fruits and derivatives. J. Food Compos. Anal. 2008, 21, 282–290. [Google Scholar] [CrossRef]
- Pinlaor, S.; Yongvanit, P.; Prakobwong, S.; Kaewsamut, B.; Khoontawad, J.; Pinlaor, P.; Hiraku, Y. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant-antioxidant status in hamsters infected with Opisthorchis viverrini. Mol. Nutr. Food Res. 2009, 53, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Donpunha, W.; Pakdeechote, P.; Kukongviriyapan, V.; Panangpetch, P.; Sripui, J.; Sae-Eaw, A. Effect of Mamao pomace on the reduction of blood pressure in l-NAME-induced hypertensive rats. Srinagarind Med. J. 2013, 28, 266–270. [Google Scholar]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Somparn, N.; Kukongviriyapan, U.; Tassaneeyakul, W.; Jetsrisuparb, A.; Kukongviriyapan, V. Modification of CYP2E1 and CYP3A4 activities in haemoglobin E-beta thalassemia patients. Eur. J. Clin. Pharmacol. 2007, 63, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. Comparison of the total phenolic content, total anthocyanin content and antioxidant activity of polyphenol-rich fruits grown in Chile. Cien. Inv. Agr. 2014, 41, 49–60. [Google Scholar] [CrossRef]
- Rimpara, Z.; Toromanovic, J.; Tahirocvic, I.; Sapcanin, A.; Sofic, E. Total content of phenols and anthocyanins in edible fruits from Bosnia. Bosnian J. Basic Med. Sci. 2007, 7, 119–122. [Google Scholar]
- Shindo, M.; Kasai, T.; Abe, A.; Kondo, Y. Effects of dietary administration of plant-derived anthocyanin-rich colors to spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2007, 53, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Cimino, F.; Saija, A.; Canali, R.; Virgili, F. Bioavailability and molecular activities of anthocyanins as modulators of endothelial function. Genes Nutr. 2014, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Shu, L.; Khor, T.O.; Lee, J.H.; Fuentes, F.; Kong, A.N. A perspective on dietary phytochemicals and cancer chemoprevention: Oxidative stress, Nrf2, and epigenomics. Top. Curr. Chem. 2013, 329, 133–162. [Google Scholar] [PubMed]
- Surh, Y.J.; Na, H.K. NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008, 2, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Warin, L.; Michel, J.B. Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J. Clin. Investig. 1992, 90, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Egashira, K.; Kataoka, C.; Usui, M.; Koyanagi, M.; Takemoto, M.; Takeshita, A. Chronic inhibition of nitric oxide synthesis in rats increases aortic superoxide anion production via the action of angiotensin II. J. Hypertens. 2000, 18, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Zalba, G.; San Jose, G.; Moreno, M.U.; Fortuno, M.A.; Fortuno, A.; Beaumont, F.J.; Diez, J. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension 2001, 38, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Schaad, N.C.; Osterheld, M.C.; Grouzmann, E.; Nussberger, J.; Brunner, H.R.; Waeber, B. Effects of chronic NO synthase inhibition in rats on renin-angiotensin system and sympathetic nervous system. Am. J. Physiol. 1995, 268, H2267–H2273. [Google Scholar] [PubMed]
- Simko, F.; Pechanova, O.; Pelouch, V.; Krajcirovicova, K.; Celec, P.; Palffy, R.; Bednarova, K.; Vrankova, S.; Adamcova, M.; Paulis, L. Continuous light and l-NAME-induced left ventricular remodelling: different protection with melatonin and captopril. J. Hypertens. 2010, 28, S13–S18. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukongviriyapan, U.; Kukongviriyapan, V.; Pannangpetch, P.; Donpunha, W.; Sripui, J.; Sae-Eaw, A.; Boonla, O. Mamao Pomace Extract Alleviates Hypertension and Oxidative Stress in Nitric Oxide Deficient Rats. Nutrients 2015, 7, 6179-6194. https://doi.org/10.3390/nu7085275
Kukongviriyapan U, Kukongviriyapan V, Pannangpetch P, Donpunha W, Sripui J, Sae-Eaw A, Boonla O. Mamao Pomace Extract Alleviates Hypertension and Oxidative Stress in Nitric Oxide Deficient Rats. Nutrients. 2015; 7(8):6179-6194. https://doi.org/10.3390/nu7085275
Chicago/Turabian StyleKukongviriyapan, Upa, Veerapol Kukongviriyapan, Patchareewan Pannangpetch, Wanida Donpunha, Jintana Sripui, Amporn Sae-Eaw, and Orachorn Boonla. 2015. "Mamao Pomace Extract Alleviates Hypertension and Oxidative Stress in Nitric Oxide Deficient Rats" Nutrients 7, no. 8: 6179-6194. https://doi.org/10.3390/nu7085275
APA StyleKukongviriyapan, U., Kukongviriyapan, V., Pannangpetch, P., Donpunha, W., Sripui, J., Sae-Eaw, A., & Boonla, O. (2015). Mamao Pomace Extract Alleviates Hypertension and Oxidative Stress in Nitric Oxide Deficient Rats. Nutrients, 7(8), 6179-6194. https://doi.org/10.3390/nu7085275