Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals
Abstract
:1. Introduction
2. Etiology of Obesity and Associated Metabolic Complications
2.1. Comorbidities Related to Obesity
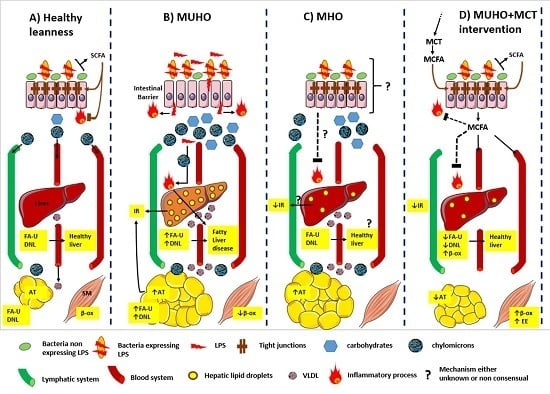
2.2. Metabolically Healthy but Obese Individuals (MHO)
3. The Gut Microbiota: A Determining Factor for Metabolic State
3.1. Gut Microbiota Dysbiosis in Obesity
3.2. Correlation of MHO Metabolic Status with Gut Microbiota Profile
4. MHO and MUHO: From Classical Dietary Interventions to a MCT-Based One?
4.1. Weight Management in MHO Subjects
4.2. The Metabolic Protective Potential of Medium Chain Triglycerides (MCTs)
4.2.1. MCTs as Bioactive Lipids
4.2.2. MCT-Supplemented Diets Prevent Obesity
(i) MCT and MCFA Exert Antilipogenic Effects
(ii) The Cardiometabolic Protective Effects of Dietary MCT
4.2.3. MCT-Supplemented Diets Improve Gut Microbiota and Intestinal Health
5. Synthesis and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, K.B.; Smith, M.S. Obesity statistics. Prim. Care 2016, 43, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Graffy, P.M.; Pickhardt, P.J. Quantification of hepatic and visceral fat by ct and mr imaging: Relevance to the obesity epidemic, metabolic syndrome and nafld. Br. J. Radiol. 2016, 89, 20151024. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hryhorczuk, C.; Sharma, S.; Fulton, S.E. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Reuter, C.P.; Silva, P.T.; Renner, J.D.; Mello, E.D.; Valim, A.R.; Pasa, L.; Silva, R.D.; Burgos, M.S. Dyslipidemia is associated with unfit and overweight-obese children and adolescents. Arq. Brasil. Cardiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Elmaogullari, S.; Tepe, D.; Ucakturk, S.A.; Karaca Kara, F.; Demirel, F. Prevalence of dyslipidemia and associated factors in obese children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shore, S.A. Obesity, asthma, and the microbiome. Physiology 2016, 31, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Amoli, M.M.; Qorbani, M.; Hasani-Ranjbar, S. Adiponectin gene variants and abdominal obesity in an iranian population. Eat. Weight Disord. 2016. [Google Scholar] [CrossRef] [PubMed]
- Waleh, M.Q. Impacts of physical activity on the obese. Prim. Care 2016, 43, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M. Circadian clocks, obesity and cardiometabolic function. Diabetes Obes. Metab. 2015, 17, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, L.; Sjodin, A.; Holmback, U.; Astrup, A.; Chaput, J.P. Short sleep duration and its association with energy metabolism. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2012, 13, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.M.; Tan, T.M.; Bloom, S.R. Gastrointestinal hormones and their role in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Porta, M.; Jacobs, D.R., Jr.; Vandenberg, L.N. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 2014, 35, 557–601. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Perez de Heredia, F.; Gomez-Martinez, S.; Marcos, A. The role of probiotics on the microbiota: Effect on obesity. Nutr. Clin. Pract. 2016. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Almiron-Roig, E. Frontiers in neuroscience human perceptions and preferences for fat-rich foods. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., Le Coutre, J., Eds.; CRC Press/Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2010. [Google Scholar]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabol. Clin. Exp. 2014, 63, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Feldstein, A.E. Novel molecular mechanisms in the development of non-alcoholic steatohepatitis. Diabetes Metabol. J. 2016, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaila, B.; Raman, M. Obesity: A review of pathogenesis and management strategies. Can. J. Gastroenterol. 2008, 22, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Rankinen, T.; Zuberi, A.; Chagnon, Y.C.; Weisnagel, S.J.; Argyropoulos, G.; Walts, B.; Perusse, L.; Bouchard, C. The human obesity gene map: The 2005 update. Obesity 2006, 14, 529–644. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Travisano, M.; Tahiliani, K.G.; Rached, M.T.; Mirza, S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. 2008, 32, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- McAllister, E.J.; Dhurandhar, N.V.; Keith, S.W.; Aronne, L.J.; Barger, J.; Baskin, M.; Benca, R.M.; Biggio, J.; Boggiano, M.M.; Eisenmann, J.C.; et al. Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr. 2009, 49, 868–913. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, T.; Kobayashi, N.; Mamiya, S.; Nakanishi, T.; Nishikawa, J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol. Pharmacol. 2005, 67, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Maachi, M.; Pieroni, L.; Bruckert, E.; Jardel, C.; Fellahi, S.; Hainque, B.; Capeau, J.; Bastard, J.P. Systemic low-grade inflammation is related to both circulating and adipose tissue tnfalpha, leptin and il-6 levels in obese women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.A.; Kaouache, M.; Rempel, P.; Joseph, L.; Dawes, M.; Lau, D.C.; Lowensteyn, I. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: A modelling study. Lancet Diabetes Endocrinol. 2015, 3, 114–122. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [PubMed]
- Kyrou, I.; Randeva, H.S.; Weickert, M.O. Clinical problems caused by obesity. In Endotext; De Groot, L.J., Beck-Peccoz, P., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Koch, C., McLachlan, R., New, M., Rebar, R., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 american heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Dixit, V.D.; Kahn, C.R.; Leibel, R.L.; Lin, X.; Nieuwdorp, M.; Pietilainen, K.H.; Rabasa-Lhoret, R.; Roden, M.; Scherer, P.E.; et al. Metabolically healthy and unhealthy obese—The 2013 stock conference report. Obes. Rev. 2014, 15, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D. Metabolically healthy but obese individuals. Lancet 2008, 372, 1281–1283. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Metabolically healthy obesity: Different prevalences using different criteria. Eur. J. Clin. Nutr. 2010, 64, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Plourde, G.; Karelis, A.D. Current issues in the identification and treatment of metabolically healthy but obese individuals. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J. Integrating publicly available genome-wide association data to study the genetic basis of metabolically healthy obese and metabolically obese but normal-weight individuals. Diabetes 2014, 63, 4004–4007. [Google Scholar] [CrossRef] [PubMed]
- Berezina, A.; Belyaeva, O.; Berkovich, O.; Baranova, E.; Karonova, T.; Bazhenova, E.; Brovin, D.; Grineva, E.; Shlyakhto, E. Prevalence, risk factors, and genetic traits in metabolically healthy and unhealthy obese individuals. Biomed. Res. Int. 2015, 2015, 548734. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, A.D.; Sung, M.M.; Boisvenue, J.J.; Barr, A.J.; Dyck, J.R. Adiponectin gene therapy ameliorates high-fat, high-sucrose diet-induced metabolic perturbations in mice. Nutr. Diabetes 2012, 2, e45. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.Y.; Lee, M.S. Gut microbiota and metabolic disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Lee, S.M.; Shen, Y.; Khosravi, A.; Mazmanian, S.K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 2010, 107, 243–274. [Google Scholar] [PubMed]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial hif augments tissue barrier function. Cell Host Microb. 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Muller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chassaing, B.; Zhang, L.; San Yeoh, B.; Xiao, X.; Kumar, M.; Baker, M.T.; Cai, J.; Walker, R.; Borkowski, K.; et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl coa desaturase 1 (scd1) promotes metabolic syndrome in tlr5-deficient mice. Cell Metab. 2015, 22, 983–996. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.; Kersten, S. The role of the gut microbiota in metabolic health. Fed. Am. Soc. Exp. Biol. J. 2015, 29, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferrieres, J. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [PubMed]
- Maron, D.F.; Smith, T.J.S.; Nachman, K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Cazer, C.L.; Volkova, V.V.; Grohn, Y.T. Use of pharmacokinetic modeling to assess antimicrobial pressure on enteric bacteria of beef cattle fed chlortetracycline for growth promotion, disease control, or treatment. Foodborne Pathog. Dis. 2014, 11, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Stedtfeld, R.D.; Wang, Q.; Cole, J.R.; Hashsham, S.A.; Looft, T.; Zhu, Y.G.; Tiedje, J.M. Clusters of antibiotic resistance genes enriched together stay together in swine agriculture. mBio 2016. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving glp-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N. Obesity, fatty liver disease and intestinal microbiota. World J. Gastroenterol. 2014, 20, 16452–16463. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Crosstalk between the gut microbiota and the endocannabinoid system: Impact on the gut barrier function and the adipose tissue. Clin. Microbiol. Infect. 2012, 18, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Membrez, M.; Blancher, F.; Jaquet, M.; Bibiloni, R.; Cani, P.D.; Burcelin, R.G.; Corthesy, I.; Mace, K.; Chou, C.J. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. Fed. Am. Soc. Exp. Biol. J. 2008, 22, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-grade inflammation, obesity, and diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Abdallah Ismail, N.; Ragab, S.H.; Abd Elbaky, A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of firmicutes and bacteroidetes in gut microbiota in obese and normal weight egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microb. 2012, 3, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Backhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Fact. 2011, 10, S10. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M. Gut microbiota and energy balance: Role in obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Berge, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Van Hee, V.F.; Piront, N.; De Backer, F.; Toussaint, O.; Cani, P.D.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2012, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Stahlman, M.; Ilkayeva, O.; Arnemo, J.M.; Kindberg, J.; Josefsson, J.; Newgard, C.B.; Frobert, O.; Backhed, F. The gut microbiota modulates energy metabolism in the hibernating brown bear Ursus arctos. Cell Rep. 2016, 14, 1655–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arinell, K.; Sahdo, B.; Evans, A.L.; Arnemo, J.M.; Baandrup, U.; Frobert, O. Brown bears (Ursus arctos) seem resistant to atherosclerosis despite highly elevated plasma lipids during hibernation and active state. Clin. Transl. Sci. 2012, 5, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Mar Rodriguez, M.; Perez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jove, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.T.; Wing, R.R.; Hill, J.O. The prevalence of weight loss maintenance among american adults. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Malspeis, S.; Willett, W.C. Weight cycling and mortality among middle-aged or older women. Arch. Intern. Med. 2009, 169, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Hamm, P.; Shekelle, R.B.; Stamler, J. Large fluctuations in body weight during young adulthood and twenty-five-year risk of coronary death in men. Am. J. Epidemiol. 1989, 129, 312–318. [Google Scholar] [PubMed]
- Chevrier, J.; Dewailly, E.; Ayotte, P.; Mauriege, P.; Despres, J.P.; Tremblay, A. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Hue, O.; Marcotte, J.; Berrigan, F.; Simoneau, M.; Dore, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Increased plasma levels of toxic pollutants accompanying weight loss induced by hypocaloric diet or by bariatric surgery. Obes. Surg. 2006, 16, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Marchand, P.; Henegar, C.; Antignac, J.P.; Alili, R.; Poitou, C.; Bouillot, J.L.; Basdevant, A.; Le Bizec, B.; Barouki, R.; et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ. Health Perspect. 2011, 119, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Machann, J.; Schick, F.; Rittig, K.; Machicao, F.; Fritsche, A.; Haring, H.U.; Stefan, N. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 2011, 54, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.J.; Hyun, Y.J.; Kim, O.Y.; Kim, J.Y.; Jang, Y.; Lee, J.H. Weight loss effect on inflammation and ldl oxidation in metabolically healthy but obese (mho) individuals: Low inflammation and ldl oxidation in mho women. Int. J. Obes. 2006, 30, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Abbasi, F.; Lamendola, C.; Liang, L.; Reaven, G.; Schaaf, P.; Reaven, P. Differentiation between obesity and insulin resistance in the association with c-reactive protein. Circulation 2002, 106, 2908–2912. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, P.M.; Ross, R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010, 33, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Truesdale, K.P.; Bradshaw, P.T.; Cai, J.; Stevens, J. Three-year weight change and cardiometabolic risk factors in obese and normal weight adults who are metabolically healthy: The atherosclerosis risk in communities study. Int. J. Obes. 2015, 39, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Dalzill, C.; Nigam, A.; Juneau, M.; Guilbeault, V.; Latour, E.; Mauriege, P.; Gayda, M. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can. J. Cardiol. 2014, 30, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Wharton, S.; Sharma, A.M.; Ardern, C.I.; Kuk, J.L. Influence of a clinical lifestyle-based weight loss program on the metabolic risk profile of metabolically normal and abnormal obese adults. Obesity 2013, 21, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Folli, F.; Perego, L.; Hribal, M.L.; Pontiroli, A.E. Effects of weight loss in metabolically healthy obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. PLoS ONE 2011, 6, e17737. [Google Scholar] [CrossRef] [PubMed]
- Gilardini, L.; Vallone, L.; Cottafava, R.; Redaelli, G.; Croci, M.; Conti, A.; Pasqualinotto, L.; Invitti, C. Insulin sensitivity deteriorates after short-term lifestyle intervention in the insulin sensitive phenotype of obesity. Obes. Facts 2012, 5, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Messier, V.; Brochu, M.; Rabasa-Lhoret, R. Metabolically healthy but obese women: Effect of an energy-restricted diet. Diabetologia 2008, 51, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, P.; Tzotzas, T.; Christou, G.; Elisaf, M.S.; Kiortsis, D.N. Does the presence of metabolic syndome influence weight loss in obese and overweight women? Metab. Syndr. Relat. Disord. 2010, 8, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Bioactive lipids in metabolic syndrome. Prog. Lipid Res. 2008, 47, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Medium-chain fatty acids: Functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Babayan, V.K. Medium chain triglycerides and structured lipids. Lipids 1987, 22, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [PubMed]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to december 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Papamandjaris, A.A.; MacDougall, D.E.; Jones, P.J. Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications. Life Sci. 1998, 62, 1203–1215. [Google Scholar] [CrossRef]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Noguchi, O.; Takeuchi, H.; Kubota, F.; Tsuji, H.; Aoyama, T. Larger diet-induced thermogenesis and less body fat accumulation in rats fed medium-chain triacylglycerols than in those fed long-chain triacylglycerols. J. Nutr. Sci. Vitaminol. 2002, 48, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Akpa, M.M.; Point, F.; Sawadogo, S.; Radenne, A.; Mounier, C. Inhibition of insulin and t3-induced fatty acid synthase by hexanoate. Lipids 2010, 45, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Li, Q.; Rayalam, S.; Hartzell, D.L.; Della-Fera, M.A.; Hamrick, M.W.; Baile, C.A. Central leptin versus ghrelin: Effects on bone marrow adiposity and gene expression. Endocrine 2010, 37, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239. [Google Scholar] [PubMed]
- Goodridge, A.G.; Thurmond, D.C.; Baillie, R.A.; Hodnett, D.W.; Xu, G. Nutritional and hormonal regulation of the gene for malic enzyme. Z. Ernahrungswissenschaft 1998, 37, 8–13. [Google Scholar]
- Roncero, C.; Goodridge, A.G. Hexanoate and octanoate inhibit transcription of the malic enzyme and fatty acid synthase genes in chick embryo hepatocytes in culture. J. Biol. Chem. 1992, 267, 14918–14927. [Google Scholar] [PubMed]
- Thurmond, D.C.; Baillie, R.A.; Goodridge, A.G. Regulation of the action of steroid/thyroid hormone receptors by medium-chain fatty acids. J. Biol. Chem. 1998, 273, 15373–15381. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fu, J.; Li, L.; Gong, D.; Wen, X.; Yu, P.; Zeng, Z. Medium-chain fatty acid reduces lipid accumulation by regulating expression of lipid-sensing genes in human liver cells with steatosis. Int. J. Food Sci. Nutr. 2016, 67, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Lefevre, A.; Spritz, N.; Feinman, L.; DeCarli, L.M. Difference in hepatic metabolism of long- and medium-chain fatty acids: The role of fatty acid chain length in the production of the alcoholic fatty liver. J. Clin. Investig. 1967, 46, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.R.; Hamawy, K.J.; Moldawer, L.L.; Istfan, N.; Bistrian, B.R.; Blackburn, G.L. Evaluation of the protein quality of diets containing medium- and long-chain triglyceride in healthy rats. J. Nutr. 1986, 116, 343–349. [Google Scholar] [PubMed]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Hill, J.O.; Peters, J.C.; Yang, D.; Sharp, T.; Kaler, M.; Abumrad, N.N.; Greene, H.L. Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metab. Clin. Exp. 1989, 38, 641–648. [Google Scholar] [CrossRef]
- Seaton, T.B.; Welle, S.L.; Warenko, M.K.; Campbell, R.G. Thermic effect of medium-chain and long-chain triglycerides in man. Am. J. Clin. Nutr. 1986, 44, 630–634. [Google Scholar] [PubMed]
- Scalfi, L.; Coltorti, A.; Contaldo, F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. Am. J. Clin. Nutr. 1991, 53, 1130–1133. [Google Scholar] [PubMed]
- Dulloo, A.G.; Fathi, M.; Mensi, N.; Girardier, L. Twenty-four-hour energy expenditure and urinary catecholamines of humans consuming low-to-moderate amounts of medium-chain triglycerides: A dose-response study in a human respiratory chamber. Eur. J. Clin. Nutr. 1996, 50, 152–158. [Google Scholar] [PubMed]
- Ishizawa, R.; Masuda, K.; Sakata, S.; Nakatani, A. Effects of different fatty acid chain lengths on fatty acid oxidation-related protein expression levels in rat skeletal muscles. J. Oleo Sci. 2015, 64, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Binnert, C.; Pachiaudi, C.; Beylot, M.; Hans, D.; Vandermander, J.; Chantre, P.; Riou, J.P.; Laville, M. Influence of human obesity on the metabolic fate of dietary long- and medium-chain triacylglycerols. Am. J. Clin. Nutr. 1998, 67, 595–601. [Google Scholar] [PubMed]
- Nagata, J.; Kasai, M.; Watanabe, S.; Ikeda, I.; Saito, M. Effects of highly purified structured lipids containing medium-chain fatty acids and linoleic acid on lipid profiles in rats. Biosci. Biotechnol. Biochem. 2003, 67, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Nagata, J.; Kasai, M.; Negishi, S.; Saito, M. Effects of structured lipids containing eicosapentaenoic or docosahexaenoic acid and caprylic acid on serum and liver lipid profiles in rats. BioFactors 2004, 22, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Matsuo, M.; Kasai, M.; Takeuchi, H. Effects of a liquid diet supplement containing structured medium- and long-chain triacylglycerols on bodyfat accumulation in healthy young subjects. Asia Pac. J. Clin. Nutr. 2001, 10, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Kasai, M.; Takeuchi, H.; Nakamura, M.; Okazaki, M.; Kondo, K. Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J. Nutr. 2001, 131, 2853–2859. [Google Scholar] [PubMed]
- St-Onge, M.P.; Mayrsohn, B.; O’Keeffe, M.; Kissileff, H.R.; Choudhury, A.R.; Laferrere, B. Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur. J. Clin. Nutr. 2014, 68, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ross, R.; Parsons, W.D.; Jones, P.J. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes. Res. 2003, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Lemarie, F.; Beauchamp, E.; Dayot, S.; Duby, C.; Legrand, P.; Rioux, V. Dietary caprylic acid (c8:0) does not increase plasma acylated ghrelin but decreases plasma unacylated ghrelin in the rat. PLoS ONE 2015, 10, e0133600. [Google Scholar] [CrossRef] [PubMed]
- Lemarie, F.; Beauchamp, E.; Legrand, P.; Rioux, V. Revisiting the metabolism and physiological functions of caprylic acid (c8:0) with special focus on ghrelin octanoylation. Biochimie 2016, 120, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mulholland, M.; Zhang, W. Ghrelin o-acyltransferase (goat) and energy metabolism. Sci. China Life Sci. 2016, 59, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Nosaka, N.; Maki, H.; Negishi, S.; Aoyama, T.; Nakamura, M.; Suzuki, Y.; Tsuji, H.; Uto, H.; Okazaki, M.; et al. Effect of dietary medium- and long-chain triacylglycerols (mlct) on accumulation of body fat in healthy humans. Asia Pac. J. Clin. Nutr. 2003, 12, 151–160. [Google Scholar] [PubMed]
- St-Onge, M.P.; Bourque, C.; Jones, P.J.; Ross, R.; Parsons, W.E. Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 95–102. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Jones, P.J. Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Airhart, S.; Cade, W.T.; Jiang, H.; Coggan, A.R.; Racette, S.B.; Korenblat, K.; Spearie, C.A.; Waller, S.; O’Connor, R.; Bashir, A.; et al. A diet rich in medium-chain fatty acids improves systolic function and alters the lipidomic profile in patients with type 2 diabetes: A pilot study. J. Clin. Endocrinol. Metab. 2016, 101, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Assuncao, M.L.; Ferreira, H.S.; dos Santos, A.F.; Cabral, C.R., Jr.; Florencio, T.M. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids 2009, 44, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Melchin, M.; Moehring, J.; Wagner, A.E. Polyphenols from cocoa and vascular health—A critical review. Int. J. Mol. Sci. 2009, 10, 4290–4309. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.; St-Onge, M.P.; Papamandjaris, A.A.; Cohn, J.S.; Jones, P.J. Consumption of an oil composed of medium chain triacyglycerols, phytosterols, and n-3 fatty acids improves cardiovascular risk profile in overweight women. Metabol. Clin. Exp. 2003, 52, 771–777. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Lamarche, B.; Mauger, J.F.; Jones, P.J. Consumption of a functional oil rich in phytosterols and medium-chain triglyceride oil improves plasma lipid profiles in men. J. Nutr. 2003, 133, 1815–1820. [Google Scholar] [PubMed]
- St-Onge, M.P.; Bosarge, A.; Goree, L.L.; Darnell, B. Medium chain triglyceride oil consumption as part of a weight loss diet does not lead to an adverse metabolic profile when compared to olive oil. J. Am. Coll. Nutr. 2008, 27, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2015, 115, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Nakanishi, S.; Shiomi, S.; Kiyokawa, S.; Kakimoto, S.; Nakagawa, K.; Hosoe, K.; Minami, K.; Nadamoto, T. Enhancement of fat oxidation by licorice flavonoid oil in healthy humans during light exercise. J. Nutr. Sci. Vitaminol. 2015, 61, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E. The antimicrobial function of milk lipids. Adv. Nutr. Res. 2001, 10, 271–285. [Google Scholar] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Goldblum, R.M.; Garza, C.; Goldman, A.S. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr. Res. 1986, 20, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Papavassilis, C.; Mach, K.K.; Mayser, P.A. Medium-chain triglycerides inhibit growth of malassezia: Implications for prevention of systemic infection. Crit. Care Med. 1999, 27, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Asakawa, M.; Yamamoto, M.; Matsuda, M.; Maki, A.; Matsumoto, Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann. Surg. 2003, 237, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Zentek, J.; Ferrara, F.; Pieper, R.; Tedin, L.; Meyer, W.; Vahjen, W. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J. Anim. Sci. 2013, 91, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Dierick, N.; Michiels, J.; Van Nevel, C. Effect of medium chain fatty acids and benzoic acid, as alternatives for antibiotics, on growth and some gut parameters in piglets. Commun. Agric. Appl. Biol. Sci. 2004, 69, 187–190. [Google Scholar] [PubMed]
- Jacobi, S.K.; Odle, J. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Ogiku, M.; Tsuchiya, M.; Ishii, K.; Hara, M. Enteral diets enriched with medium-chain triglycerides and n-3 fatty acids prevent chemically induced experimental colitis in rats. Transl. Res. J. Lab. Clin. Med. 2010, 156, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Ishii, K.; Hosomura, N.; Ogiku, M. Dietary medium-chain triglycerides prevent chemically induced experimental colitis in rats. Transl. Res. J. Lab. Clin. Med. 2010, 155, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.A.; Dierick, N.A. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: Concept, possibilities and limitations. An overview. Nutr. Res. Rev. 2003, 16, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Asakawa, M.; Maki, A.; Amemiya, H.; Hirai, Y.; Matsuda, M.; Yamamoto, M. Medium-chain triglycerides enhance secretory iga expression in rat intestine after administration of endotoxin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G1081–G1089. [Google Scholar] [CrossRef] [PubMed]
- Servier Medical Art (Servier). Available online: http://www.servier.fr/smart/banque-dimages-powerpoint/ (acessed on 21 March 2016).

| Model | Main Reported Effects for MCT or MCFA | References |
|---|---|---|
| Hepatocyte | Downregulated expression of genes involved in DNL and fatty acid uptake; promoted lipid catabolism; reduced steatosis; prevented deleterious lipid accumulation | [108,113,115] |
| Rat | Lowered TG accumulation in the liver; reduced alcoholic steatosis | [116] |
| Rat | Decreased body weight gain and body fat mass; lowered fat accumulation and visceral adiposity; did not affect protein assimilation nor metabolism | [107,117] |
| Rat | Resulted in a higher induction of oxygen consumption and thermogenesis | [107] |
| Human | Significantly increased postprandial oxygen consumption, energy expenditure, and fat oxidation, in a MCT dose-dependent manner and at a greater extend for lower BMIs | [119,120,121,122,135,136,142,144] |
| Human | Decreased global adiposity, body fat, and whole-body subcutaneous adipose tissue loss, waist circumference; significantly lowered rate of variation of body fat percentage | [127,130,134] |
| Human | Did not improve global adiposity | [135] |
| Human | Did not elevate postprandial circulating TG; did not modulate glucose response, insulinemia and circulating TG levels; lowered LDL/HDL ratio, total and HDL-cholesterol; improved cardiometabolic profile | [129,134,136,138,141] |
| Human | Promoted rise in leptin and peptide YY | [129] |
| Model | Main Reported Effects | References |
|---|---|---|
| Malassezia | Supressed growth of M. sympodialis and M. furfur | [148] |
| Rats | Prevented acute LPS administration-induced mortality, liver injury, liver inflammation, gut impermeability and injury; blunted LPS-induced endotoxemia | [149] |
| Rats | Significantly blunted TNBS-induced colitis; improved both colonic MPO activity and colonocytes-expressed inflammatory markers | [155] |
| Rats | Improved gut integrity; modulated immune response to LPS; improved intestinal secretion of IgA | [158] |
| Piglets | Lowered intestinal pH, in synergy with OA; modulated several gut microbial taxa, potentially preventing postweaning diarrhea | [151] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial, S.A.; Karelis, A.D.; Bergeron, K.-F.; Mounier, C. Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients 2016, 8, 281. https://doi.org/10.3390/nu8050281
Rial SA, Karelis AD, Bergeron K-F, Mounier C. Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients. 2016; 8(5):281. https://doi.org/10.3390/nu8050281
Chicago/Turabian StyleRial, Sabri Ahmed, Antony D. Karelis, Karl-F. Bergeron, and Catherine Mounier. 2016. "Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals" Nutrients 8, no. 5: 281. https://doi.org/10.3390/nu8050281
APA StyleRial, S. A., Karelis, A. D., Bergeron, K. -F., & Mounier, C. (2016). Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients, 8(5), 281. https://doi.org/10.3390/nu8050281