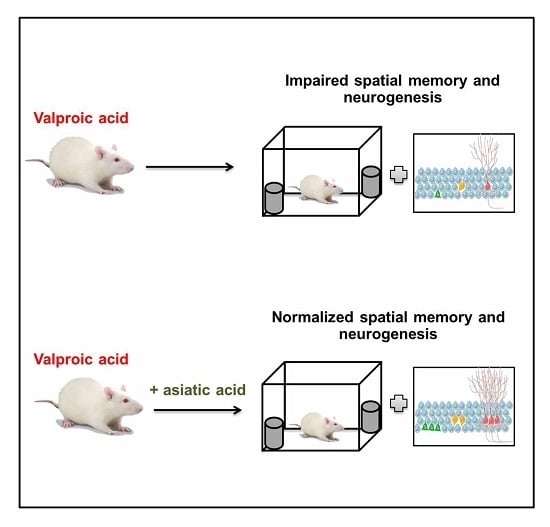
Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs Administration
2.3. Novel Object Location (NOL) Test
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Asiatic Acid Prevents the Spatial Working Memory Deficits Caused by VPA
3.2. Asiatic Acid Prevents the Reduction in Cell Proliferation in the SGZ Caused by VPA
3.3. Asiatic Acid Prevents the Reduction in Cell Survival in SGZ Caused by VPA
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henry, T.R. The history of valproate in clinical neuroscience. Psychopharmacol. Bull. 2003, 37 (Suppl. S2), 5–16. [Google Scholar] [PubMed]
- Johannessen, C.U. Mechanisms of action of valproate: A commentatory. Neurochem. Int. 2000, 37, 103–110. [Google Scholar] [CrossRef]
- Perucca, E. Pharmacological and therapeutic properties of valproate: A summary after 35 years of clinical experience. CNS Drugs 2002, 16, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Nakashima, K.; Clemenson, G.D.; Mejia, E.; Mathews, E.; Ure, K.; Ogawa, S.; Sinton, C.M.; Gage, F.H.; Hsieh, J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J. Neurosci. 2007, 27, 5967–5975. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Gastens, A.M.; Sun, M.Z.; Hausknecht, M.; Loscher, W. Treatment with valproate after status epilepticus: Effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology 2006, 51, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [CrossRef] [PubMed]
- Umka, J.; Mustafa, S.; ElBeltagy, M.; Thorpe, A.; Latif, L.; Bennett, G.; Wigmore, P.M. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 2010, 166, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Senturk, V.; Goker, C.; Bilgic, A.; Olmez, S.; Tugcu, H.; Oncu, B.; Atbasoglu, E.C. Impaired verbal memory and otherwise spared cognition in remitted bipolar patients on monotherapy with lithium or valproate. Bipolar Disord. 2007, 9 (Suppl. S1), 136–144. [Google Scholar] [CrossRef] [PubMed]
- Nadebaum, C.; Anderson, V.; Vajda, F.; Reutens, D.; Barton, S.; Wood, A. The Australian brain and cognition and antiepileptic drugs study: IQ in school-aged children exposed to sodium valproate and polytherapy. J. Int. Neuropsychol. Soc. 2011, 17, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Calandre, E.P.; Dominguez-Granados, R.; Gomez-Rubio, M.; Molina-Font, J.A. Cognitive effects of long-term treatment with phenobarbital and valproic acid in school children. Acta Neurol. Scand. 1990, 81, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.D.; Oleinik, O.E.; Glauser, T.A.; Maertens, P.; Eder, D.N.; Pippenger, C.E. Altered antioxidant enzyme activities in children with a serious adverse experience related to valproic acid therapy. Neuropediatrics 1998, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Seckin, S.; Basaran-Kucukgergin, C.; Uysal, M. Effect of acute and chronic administration of sodium valproate on lipid peroxidation and antioxidant system in rat liver. Pharmacol. Toxicol. 1999, 85, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Kam, M.; Nannmark, U.; Anderson, M.F.; Axell, M.Z.; Wikkelso, C.; Holtas, S.; van Roon-Mom, W.M.; Bjork-Eriksson, T.; Nordborg, C.; et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007, 315, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Toni, N.; Laplagne, D.A.; Zhao, C.; Lombardi, G.; Ribak, C.E.; Gage, F.H.; Schinder, A.F. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008, 11, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. Adult neurogenesis in the mammalian central nervous system: Functionality and potential clinical interest. Med. Sci. Monit. 2005, 11, RA247–RA252. [Google Scholar] [PubMed]
- Van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- ELBeltagy, M.; Mustafa, S.; Umka, J.; Lyons, L.; Salman, A.; Chur-yoe, G.T.; Bhalla, N.; Bennett, G.; Wigmore, P.M. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav. Brain Res. 2010, 208, 112–117. [Google Scholar] [CrossRef] [PubMed]
- ELBeltagy, M.; Mustafa, S.; Umka, J.; Lyons, L.; Salman, A.; Dormon, K.; Allcock, C.; Bennett, G.; Wigmore, P. The effect of 5-fluorouracil on the long term survival and proliferation of cells in the rat hippocampus. Brain Res. Bull. 2012, 88, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Kuhn, H.G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997, 386, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Ko, I.G.; Kim, B.K.; Shin, M.S.; Cho, S.; Kim, C.J.; Kim, S.H.; Baek, S.S.; Lee, E.K.; Jee, Y.S. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp. Gerontol. 2010, 45, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Perfilieva, E.; Johansson, U.; Orwar, O.; Eriksson, P.S. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 1999, 39, 569–578. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.G.; Halabisky, B.; Zhou, Y.G.; Palop, J.J.; Yu, G.Q.; Mucke, L.; Gan, L. Imbalance between gabaergic and glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer’s disease. Cell Stem Cell 2009, 5, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.G.; Senut, M.C.; Zemke, D.; Min, J.; Frenkel, M.B.; Greenberg, E.J.; Yu, S.W.; Ahn, N.; Goudreau, J.; Kassab, M.; et al. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J. Neurosci. Res. 2009, 87, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Dou, Y.; Xia, B.; Rong, W.; Rimbach, G.; Lou, Y. Asiatic acid protects primary neurons against C2-ceramide-induced apoptosis. Eur. J. Pharmacol. 2012, 679, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.N.; Abdullah, J.; Habsah, M.; Ghani, R.I.; Rammes, G. Inhibitory effect of asiatic acid on acetylcholinesterase, excitatory post synaptic potential and locomotor activity. Phytomedicine 2012, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.N.; Habsah, M.; Zamzuri, I.; Rammes, G.; Hasnan, J.; Abdullah, J. Effects of asiatic acid on passive and active avoidance task in male Spraque-Dawley rats. J. Ethnopharmacol. 2011, 134, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Chiu, C.S.; Chen, H.J.; Hou, W.C.; Sheu, M.J.; Lin, Y.C.; Shie, P.H.; Huang, G.J. Antinociceptive activities and the mechanisms of anti-inflammation of asiatic acid in mice. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.H.V.; Gupta, Y.K. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin. Exp. Pharmacol. Physoil. 2003, 30, 336–342. [Google Scholar] [CrossRef]
- Jayashree, G.; Kurup Muraleedhara, G.; Sudarslal, S.; Jacob, V.B. Anti-oxidant activity of Centella asiatica on lymphoma-bearing mice. Fitoterapia 2003, 74, 431–434. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, S.R.; Sung, S.H.; Lim, D.; Kim, H.; Choi, H.; Park, H.K.; Je, S.; Ki, Y.C. Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res. Commun. Mol. Pathol. Pharmacol. 2000, 108, 75–86. [Google Scholar] [PubMed]
- Ramachandran, V.; Saravanan, R. Efficacy of asiatic acid, a pentacyclic triterpene on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2013, 20, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.F.; Xiong, Y.Y.; Liu, J.K.; Qian, J.J.; Zhu, L.; Gao, J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol. Sin. 2012, 33, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Leksomboon, R.; Chaichun, A.; Wigmore, P.; Welbat, J.U. Effects of asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Nutrients 2015, 7, 8413–8423. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Koo, K.A.; Lee, M.K.; Park, H.G.; Jew, S.S.; Cha, K.H.; Kim, Y.C. Asiatic acid derivatives enhance cognitive performance partly by improving acetylcholine synthesis. J. Pharm. Pharmacol. 2004, 56, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Centella asiatica (L.) urban: From traditional medicine to modern medicine with neuroprotective potential. Evid. Based Complement. Altern. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef] [PubMed]
- Dix, S.L.; Aggleton, J.P. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Lyons, L.; ElBeltagy, M.; Umka, J.; Markwick, R.; Startin, C.; Bennett, G.; Wigmore, P. Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology 2011, 215, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Burton, G.J. Methodological problems in placental morphometry: Apologia for the use of stereology based on sound sampling practice. Placenta 1988, 9, 565–581. [Google Scholar] [CrossRef]
- Weible, A.P.; Rowland, D.C.; Pang, R.; Kentros, C. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J. Neurophysiol. 2009, 102, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Michalikova, S.; Bradford, A.; Ahmed, S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav. Brain Res. 2005, 159, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Walker, A.; Bennett, G.; Wigmore, P.M. 5-fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur. J. Neurosci. 2008, 28, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Endl, E.; Gerdes, J. The Ki-67 protein: Fascinating forms and an unknown function. Exp. Cell Res. 2000, 257, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef]
- Wojtowicz, J.M.; Kee, N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006, 1, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Cooper-Kuhn, C.M. Bromodeoxyuridine and the detection of neurogenesis. Curr. Pharm. Biotechnol. 2007, 8, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.L.; Hazzalin, C.A.; Mahadevan, L.C. Enhanced histone acetylation and transcription: A dynamic perspective. Mol. Cell 2006, 23, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kuwagata, M.; Ogawa, T.; Shioda, S.; Nagata, T. Observation of fetal brain in a rat valproate-induced autism model: A developmental neurotoxicity study. Int. J. Dev. Neurosci. 2009, 27, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Park, S.P.; Kwon, S.H. Cognitive effects of antiepileptic drugs. J. Clin. Neurol. 2008, 4, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Buchsbaum, R.; Weintraub, D.; Pierro, J.; Resor, S.R., Jr.; Hirsch, L.J. Patient-reported cognitive side effects of antiepileptic drugs: Predictors and comparison of all commonly used antiepileptic drugs. Epilepsy Behav. 2009, 14, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Csete, M.; Groves, A.K.; Melega, W.; Wold, B.; Anderson, D.J. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000, 20, 7370–7376. [Google Scholar] [PubMed]
- Studer, L.; Csete, M.; Lee, S.H.; Kabbani, N.; Walikonis, J.; Wold, B.; McKay, R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 2000, 20, 7377–7383. [Google Scholar] [PubMed]
- Lee, K.Y.; Bae, O.N.; Weinstock, S.; Kassab, M.; Majid, A. Neuroprotective effect of asiatic acid in rat model of focal embolic stroke. Biol. Pharm. Bull. 2014, 37, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Mook-Jung, I.; Shin, J.E.; Yun, S.H.; Huh, K.; Koh, J.Y.; Park, H.K.; Jew, S.S.; Jung, M.W. Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. J. Neurosci. Res. 1999, 58, 417–425. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umka Welbat, J.; Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Pakdeechote, P.; Sripanidkulchai, B.; Wigmore, P. Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients 2016, 8, 303. https://doi.org/10.3390/nu8050303
Umka Welbat J, Sirichoat A, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Pakdeechote P, Sripanidkulchai B, Wigmore P. Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients. 2016; 8(5):303. https://doi.org/10.3390/nu8050303
Chicago/Turabian StyleUmka Welbat, Jariya, Apiwat Sirichoat, Wunnee Chaijaroonkhanarak, Parichat Prachaney, Wanassanun Pannangrong, Poungrat Pakdeechote, Bungorn Sripanidkulchai, and Peter Wigmore. 2016. "Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival" Nutrients 8, no. 5: 303. https://doi.org/10.3390/nu8050303
APA StyleUmka Welbat, J., Sirichoat, A., Chaijaroonkhanarak, W., Prachaney, P., Pannangrong, W., Pakdeechote, P., Sripanidkulchai, B., & Wigmore, P. (2016). Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients, 8(5), 303. https://doi.org/10.3390/nu8050303