Complementary Effects of Genetic Variations in LEPR on Body Composition and Soluble Leptin Receptor Concentration after 3-Month Lifestyle Intervention in Prepubertal Obese Children
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Dietary Intervention
2.3. Anthropometric Parameters
2.4. Biochemical Measurements
2.5. DNA Isolation and Genetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Distribution of Polymorphisms in LEPR, LEP and ADIPOQ in Obese Children
3.2. Associations of Combination of Q223R and K656N LEPR Polymorphisms with Weight Loss in Obese Children after a 3-Month Intervention
3.3. Body Composition, Biochemical Parameters and Energy Intake before and after Intervention
3.4. Associations of the LEPR Q223R and K656N Polymorphisms with the Level of Adipokines and Body Composition after 3-Month Intervention
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghalandari, H.; Hosseini-Esfahani, F.; Mirmiran, P. The association of polymorphisms in leptin/leptin receptor genes and ghrelin/ghrelin receptor genes with overweight/obesity and the related metabolic disturbances: A review. Int. J. Endocrinol. Metab. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- De Luis Roman, D.; Aller, R.; Izaola, O.; Sagrado, M.G.; Conde, R. Influence of Lys656Asn polymorphism of leptin receptor gene on leptin response secondary to two hypocaloric diets: A randomized clinical trial. Ann. Nutr. Metab. 2008, 52, 209–214. [Google Scholar] [CrossRef] [PubMed]
- De Luis Roman, D.; Aller, R.; Izaola, O.; Gonzalez Sagrado, M.; Conde, R.; de la Fuente, B.; Primo, D. Effect of Lys656Asn polymorphism of leptin receptor gene on cardiovascular risk factors and serum adipokine levels after a high polyunsaturated fat diet in obese patients. J. Clin. Lab. Anal. 2015, 29, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Rudkowska, I.; Pérusse, L. Individualized weight management: What can be learned from nutrigenomics and nutrigenetics? Prog. Mol. Biol. Transl. Sci. 2012, 108, 347–382. [Google Scholar] [PubMed]
- Farooqi, I.S.; Wangensteen, T.; Collins, S.; Kimber, W.; Matarese, G.; Keogh, J.M.; Lank, E.; Bottomley, B.; Lopez-Fernandez, J.; Ferraz-Amaro, I.; et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 2007, 356, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, O.; Seidel, B.; Kiess, W.; Thiery, J.; Keller, E.; Bottuer, A.; Kratzsch, J. The soluble leptin receptor is crucial for leptin action: Evidence from clinical and experimental data. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.K.; Power-Kehoe, L.; Chua, M.; Chu, F.; Aronne, L.; Huma, Z.; Sothern, M.; Udall, J.N.; Kahle, B.; Leibel, R.L. Exonic and intronic sequence variation in the human leptin receptor gene (LEPR). Diabetes 1997, 46, 1509–1511. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Chevalley, T.; Manen, D.; Bonjour, J.P.; Rizzoli, R.; Ferrari, S. Bone mass in prepubertal boys is associated with a Gln223Arg amino acid substitution in the leptin receptor. J. Clin. Endocrinol. Metab. 2007, 92, 4380–4386. [Google Scholar] [CrossRef] [PubMed]
- Lakka, T.A.; Rankinen, T.; Weisnagel, S.J.; Chagnon, Y.C.; Lakka, H.M.; Ukkola, O.; Boulé, N.; Rice, T.; Leon, A.S.; Skinner, J.S.; et al. Leptin and leptin receptor gene polymorphisms and changes in glucose homeostasis in response to regular exercise in nondiabetic individuals: The HERITAGE family study. Diabetes 2004, 53, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Mars, M.; van Rossum, C.T.; de Graaf, C.; Hoebee, B.; de Groot, L.C.; Kok, F.J. Leptin responsiveness to energy restriction: Genetic variation in the leptin receptor gene. Obes. Res. 2004, 12, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Bašić, M.; Butorac, A.; Landeka Jurčević, I.; Bačun-Družina, V. Obesity: Genome and environment interactions. Arh. Hig. Rada Toksikol. 2012, 63, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Field, M.R.; Ordovas, J.M.; Cupples, L.A.; Defoort, C.; Lovegrove, J.A.; Drevon, C.A.; Blaak, E.E.; et al. Leptin receptor polymorphisms interact with polyunsaturated fatty acids to augment risk of insulin resistance and metabolic syndrome in adults. J. Nutr. 2010, 140, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Phillips, C.M.; Tierney, A.C.; Pérez-Martínez, P.; Defoort, C.; Helal, O.; Lairon, D.; Planells, R.; Shaw, D.I.; Lovegrove, J.A.; et al. Gene-nutrient interactions in the metabolic syndrome: Single nucleotide polymorphisms in ADIPOQ and ADIPOR1 interact with plasma saturated fatty acids to modulate insulin resistance. Am. J. Clin. Nutr. 2010, 91, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zou, D.; Zheng, L.; Chen, G.; Lu, J.; Feng, Z. Synergistic effect of LEP and LEPR gene polymorphism on body mass index in a Chinese population. Obes. Res. Clin. Pract. 2013, 7, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Moleres, A.; Rendo-Urteaga, T.; Zulet, M.A.; Marcos, A.; Campoy, C.; Garagorri, J.M.; Martínez, J.A.; Azcona-Sanjulián, M.C.; Marti, A. Obesity susceptibility loci on body mass index and weight loss in Spanish adolescents after a lifestyle intervention. J. Pediatr. 2012, 161, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Petrone, A.; Zavarella, S.; Caiazzo, A.; Leto, G.; Spoletini, M.; Potenziani, S.; Osborn, J.; Vania, A.; Buzzetti, R. The promoter region of the adiponectin gene is a determinant in modulating insulin sensitivity in childhood obesity. Obesity 2006, 14, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Mahendran, Y.; Dwivedi, O.P.; Chauhan, G.; Ghosh, S.; Marwaha, R.K.; Tandon, N.; Bharadwaj, D. Common variants of IL6, LEPR, and PBEF1 are associated with obesity in Indian children. Diabetes 2012, 61, 626–631. [Google Scholar] [CrossRef] [PubMed]
- León-Mimila, P.; Villamil-Ramírez, H.; Villalobos-Comparán, M.; Villarreal-Molina, T.; Romero-Hidalgo, S.; López-Contreras, B.; Gutiérrez-Vidal, R.; Vega-Badillo, J.; Jacobo-Albavera, L.; Posadas-Romeros, C.; et al. Contribution of common genetic variants to obesity and obesity-related traits in Mexican children and adults. PLoS ONE 2013, 8, e70640. [Google Scholar] [CrossRef]
- Répásy, J.; Bokor, S.; Erhardt, É.; Molnár, D. Association of Gln223 Arg polymorphism of the leptin receptor gene with indicators of energy expenditure in obese children. Nutrition 2014, 30, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Kuryłowicz, A.; Ambroszkiewicz, J.; Mierzejewska, E.; Chełchowska, M.; Szamotulska, K.; Weker, H.; Puzianowska-Kuźnicka, M. ADIPOQ -11377C > G polymorphism increases the risk of adipokine abnormalities and child obesity regardless of dietary intake. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Weker, H. Simple obesity in children. A study on the role of nutritional factors. Med. Wieku Rozwoj. 2006, 10, 3–191. [Google Scholar] [PubMed]
- Oblacińska, A.; Weker, H. Prevention of Obesity in Children and Adolescents; HELP-MED: Kraków, Poland, 2008. [Google Scholar]
- Dzieniszewski, J.; Szponar, L.; Szczygieł, B.; Socha, J. Scientific Foundations of Nutrition in Hospitals in Poland; National Food and Nutrition Institute: Warsaw, Poland, 2001. [Google Scholar]
- Jarosz, M.; Traczyk, I.; Rychlik, E. Energia. In Normy Żywienia dla Populacji Polskiej—Nowelizacja; Jarosz, M., Ed.; National Food and Nutrition Institute: Warsaw, Poland, 2012; pp. 18–32. [Google Scholar]
- Palczewska, I.; Niedzwiedzka, Z. Somatic development indices in children and youth of Warsaw. Med. Wieku Rozwoj. 2001, 5, 18–118. [Google Scholar] [PubMed]
- Lohman, T.G. Skinfolds and body density and their relation to body fatness: A review. Hum. Biol. 1981, 53, 181–225. [Google Scholar] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xue, Y. Pediatric obesity: Causes, symptoms, prevention and treatment. Exp. Ther. Med. 2016, 11, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zandoná, M.R.; Rodrigues, R.O.; Albiero, G.; Campagnolo, P.D.; Vitolo, M.R.; Almeida, S.; Mattevi, V.S. Polymorphisms in LEPR, PPARG and APM1 genes: Associations with energy intake and metabolic traits in young children. Arq. Bras. Endocrinol. Metab. 2013, 57, 603–611. [Google Scholar] [CrossRef]
- Komşu-Ornek, Z.; Demirel, F.; Dursun, A.; Ermiş, B.; Pişkin, E.; Bideci, A. Leptin receptor gene Gln223Arg polymorphism is not associated with obesity and metabolic syndrome in Turkish children. Turk. J. Pediatr. 2012, 54, 20–24. [Google Scholar] [PubMed]
- Ho, M.; Garnett, S.P.; Baur, L.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics 2012, 130, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Roth, C.; Menke, T.; Andler, W. Adiponectin before and after weight loss in obese children. J. Clin. Endocrinol. Metab. 2004, 89, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
- Cambuli, V.M.; Musiu, M.C.; Incani, M.; Paderi, M.; Serpe, R.; Marras, V.; Cossu, E.; Cavallo, M.G.; Mariotti, S.; Loche, S.; et al. Assessment of adiponectin and leptin as biomarkers of positive metabolic outcome after lifestyle intervention in overweight and obese children. J. Clin. Endocrinol. Metab. 2008, 93, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Weker, H.; Ambroszkiewicz, J.; Szamotulska, K.; Chełchowska, M.; Franek, E.; Laskowska-Klita, T. Alterations in markers of bone metabolism and adipokines following a 3-month lifestyle intervention induced weight loss in obese prepubertal children. Exp. Clin. Endocrinol. Diabetes 2013, 121, 498–504. [Google Scholar] [CrossRef] [PubMed]
- De Luis Roman, D.; de la Fuente, R.A.; Sagrado, M.G.; Izaola, O.; Vicente, R.C. Leptin receptor Lys656Asn polymorphism is associated with decreased leptin response and weight loss secondary to a lifestyle modification in obese patients. Arch. Med. Res. 2006, 37, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Quinton, N.D.; Lee, A.J.; Ross, R.J.; Eastell, R.; Blakemore, A.I. A single nucleotide polymorphism (SNP) in the leptin receptor is associated with BMI, fat mass and leptin levels in postmenopausal Caucasian women. Hum. Genet. 2001, 108, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Naka, I.; Yamauchi, T.; Natsuhara, K.; Kimura, R.; Nakazawa, M.; Ishida, T.; Inaoka, T.; Matsumura, Y.; Ataka, Y.; et al. The Q223R polymorphism in LEPR is associated with obesity in Pacific Islanders. Hum. Genet. 2010, 127, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.; Rankinen, T.; Chagnon, Y.; Tremblay, A.; Pérusse, L.; Bouchard, C. Polymorphisms in the leptin and leptin receptor genes in relation to resting metabolic rate and respiratory quotient in the Québec Family Study. Int. J. Obes. 2006, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Reseland, J.E.; Syversen, U.; Bakke, I.; Qvigstad, G.; Eide, L.G.; Hjertner, O.; Gordeladze, J.O.; Drevon, C.A. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J. Bone. Miner. Res. 2001, 16, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, Y.B.; Hein, G.; Chicco, A. Metabolic syndrome: Effects of n-3 PUFAs on a model of dyslipidemia, insulin resistance and adiposity. Lipids 2007, 42, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kucukkal, T.G.; Petukh, M.; Li, L.; Alexov, E. Structural and physico-chemical effects of disease and non-disease nsSNPs on proteins. Curr. Opin. Struct. Biol. 2015, 32, 18–24. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Hanley, A.J.; Ziegler, J.T.; Brown, W.M.; Haffner, S.M.; Norris, J.M.; Rotter, J.I.; Guo, X.; Chen, Y.D.; Wagenknecht, L.E.; et al. Association between ADIPOQ SNPs with plasma adiponectin and glucose homeostasis and adiposity phenotypes in the IRAS Family Study. Mol. Genet. Metab. 2012, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Karmelic, I.; Lovric, J.; Bozina, T.; Ljubić, H.; Vogrinc, Ž.; Božina, N.; Sertić, J. Adiponectin level and gene variability are obesity and metabolic syndrome markers in young population. Arch. Med. Res. 2012, 43, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, T.I.; Boutin, P.; Taylor, M.A.; Larsen, L.H.; Verdich, C.; Petersen, L.; Holst, C.; Echwald, S.M.; Dina, C.; Toubro, S.; et al. Genetic polymorphisms and weight loss in obesity: A randomised trial of hypo-energetic high- versus low-fat diets. PLoS Clin. Trials 2006, 1, e12. [Google Scholar] [CrossRef]
- Crawford, P.B.; Obarzanek, E.; Morrison, J.; Sabry, Z.I. Comparative advantage of 3-day food records over 24-h recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J. Am. Diet. Assoc. 1994, 94, 626–630. [Google Scholar] [CrossRef]

| Gene/SNP | Obese Children with Weight Loss (ΔSDS-BMI ≤ −0.5) n (%) | Obese Children without Weight Loss (ΔSDS-BMI > −0.5) n (%) | Inheritance Model | OR (95% CI) | Unadjusted p-Value | FDR Adjusted p-Value |
|---|---|---|---|---|---|---|
| LEP -2548G > A | ||||||
| Genotypes | ||||||
| GG | 23 (32.4) | 10 (34.5) | additive | – | 0.767 | 0.767 |
| GA | 31 (43.7) | 10 (34.5) | dominant | 1.10 (0.44, 2.74) | 0.840 | 0.840 |
| AA | 17 (23.9) | 9 (31.0) | recessive | 0.70 (0.27, 1.82) | 0.463 | 0.926 |
| Alleles | ||||||
| G | 77 (54.2) | 30 (51.7) | ||||
| A | 65 (45.8) | 28 (48.3) | allelic | 0.90 (0.49, 1.67) | 0.748 | 0.748 |
| LEPR Q223R A > G | ||||||
| Genotypes | ||||||
| AA | 15 (21.1) | 14 (48.3) | additive | – | 0.014 | 0.056 |
| AG | 38 (53.5) | 11 (37.9) | dominant | 3.48 (1.38, 8.78) | 0.007 | 0.035 |
| GG | 18 (25.4) | 4 (13.8) | recessive | 2.12 (0.65, 6.93) | 0.205 | 0.820 |
| Alleles | ||||||
| A | 68 (47.9) | 39 (67.2) | ||||
| G | 74 (52.1) | 19 (32.8) | allelic | 2.23 (1.18, 4.23) | 0.013 | 0.065 |
| LEPR K656N G > C | ||||||
| Genotypes | ||||||
| GG | 58 (81.7) | 19 (65.5) | additive | – | 0.206 | 0.412 |
| GC | 11 (15.5) | 10 (34.5) | dominant | 0.43 (0.16, 1.13) | 0.081 | 0.203 |
| CC | 2 (2.8) | 0 (0.0) | recessive | – | 1.000 | 1.000 |
| Alleles | ||||||
| G | 127 (89.4) | 48 (82.8) | ||||
| C | 15 (10.6) | 10 (17.2) | allelic | 0.57 (0.24, 1.35) | 0.195 | 0.488 |
| ADIPOQ -11426A > G | ||||||
| Genotypes | ||||||
| AA | 59 (83.1) | 23 (79.3) | additive | – | – | |
| AG | 12 (16.9) | 6 (20.7) | dominant | 0.78 (0.26, 2.32) | 0.655 | 1.092 |
| GG | 0 (0.0) | 0 (0.0) | recessive | – | – | |
| Alleles | ||||||
| A | 130 (91.5) | 52 (89.7) | ||||
| G | 12 (8.5) | 6 (10.3) | allelic | 0.80 (0.28, 2.24) | 0.671 | 0.839 |
| ADIPOQ -11377C > G | ||||||
| Genotypes | ||||||
| CC | 31 (43.7) | 12 (41.4) | additive | – | 0.657 | 0.876 |
| CG | 31 (43.7) | 12 (41.4) | dominant | 0.91 (0.38, 2.18) | 0.834 | 1.043 |
| GG | 9 (12.6) | 5 (17.2) | recessive | 0.70 (0.21, 2.29) | 0.540 | 0.720 |
| Alleles | ||||||
| C | 93 (65.5) | 36 (62.1) | ||||
| G | 49 (34.5) | 22 (37.9) | allelic | 0.86 (0.46, 1.62) | 0.646 | 1.077 |
| LEPR K656N n (%) | Total | ||||
|---|---|---|---|---|---|
| GG | GC | CC | |||
| LEPR Q223R | AA | 18 (18.0%) | 9 (9.0%) | 2 (2.0%) | 29.0% |
| AG | 37 (37.0%) | 12 (12.0%) | – | 49.0% | |
| GG | 22 (22.0%) | – | – | 22.0% | |
| Total | 77.0% | 21.0% | 2.0% | 100.0% | |
| Variable | Children with Weight Loss (ΔSDS-BMI ≤ −0.5) n (%) | Children without Weight Loss (ΔSDS-BMI > −0.5) n (%) | OR | 95% CI | p |
|---|---|---|---|---|---|
| Q223R/K656N genotypes | |||||
| Wild-type/Wild-type | 9 (12.7%) | 9 (31%) | Ref | - | - |
| AG or GG/Wild-type | 49 (69.0%) | 10 (34.5%) | 5.09 | (1.60, 16.24) | 0.006 |
| Wild-type/GC or CC | 6 (8.5%) | 5 (17.2%) | 1.19 | (0.25, 5.60) | 0.828 |
| AG/GC | 7 (9.9%) | 5 (17.2%) | 1.53 | (0.34, 6.85) | 0.580 |
| Sex | |||||
| Male | 35 (49.3%) | 12 (41.4%) | Ref | - | - |
| Female | 36 (50.7%) | 17 (58.6%) | 0.95 | (0.36, 2.51) | 0.912 |
| Age (year) | – | 0.85 | (0.62, 1.16) | 0.301 | |
| Obese Children with Weight Loss (ΔSDS-BMI ≤ −0.5) n = 56 | Obese Children without Weight Loss (ΔSDS-BMI > −0.5) n = 20 | p (T0 vs. T0) | p (T3 vs. T3) | |||||
|---|---|---|---|---|---|---|---|---|
| T0 | T3 | p (T3 vs. T0) | T0 | T3 | p (T3 vs. T0) | |||
| Age (years) | 8.1 (6.8–9.2) | - | 8.8 (7.3–9.6) | - | 0.110 | |||
| Male (%) | 44.6 | - | 35.0 | - | 0.453 | |||
| Anthropometric parameters | ||||||||
| Height (cm) | 135 ± 10 | 137 ± 10 | <0.001 | 139 ± 10 | 141 ± 10 | <0.001 | 0.137 | 0.152 |
| SDS-height | 0.99 ± 1.01 | 1.31 ± 1.00 | <0.001 | 1.14 ± 1.08 | 1.42 ± 1.12 | <0.001 | 0.572 | 0.700 |
| Weight (kg) | 45.2 (39.6–56.5) | 42.9 (35.7–51.8) | <0.001 | 50.7 (39.7–56.6) | 50.8 (39.8–56.3) | 0.779 | 0.501 | 0.037 |
| BMI | 24.7 (23.1–28.1) | 22.6 (20.4–25.5) | <0.001 | 25.8 (23.5–27.0) | 25.2 (22.8–26.8) | 0.002 | 0.929 | 0.013 |
| SDS-BMI | 3.52 (2.71–4.81) | 2.41 (1.73–3.89) | <0.001 | 3.40 (2.88–4.02) | 3.10 (2.50–3.87) | 0.002 | 0.596 | 0.081 |
| Body composition | ||||||||
| Fat mass (%) | 41.1 (39.3–44.5) | 38.4 (33.9–41.5) | <0.001 | 39.6 (36.7–43.4) | 40.3 (37.2–43.5) | 0.911 | 0.183 | 0.253 |
| Fat mass (kg) | 19.9 (15.7–23.7) | 16.8 (13.5–20.9) | <0.001 | 20.8 (16.9–22.9) | 20.3 (16.1–24.3) | 0.881 | 0.832 | 0.031 |
| Fat-free mass (kg) | 27.5 ± 6.9 | 26.8 ± 6.5 | <0.001 | 30.3 ± 6.8 | 30.3 ± 6.7 | 0.876 | 0.130 | 0.048 |
| Biochemical measurements | ||||||||
| Leptin (ng/mL) | 37.0 (23.5–54.7) | 11.8 (5.9–26.6) | <0.001 | 42.9 (29.3–66.2) | 26.5 (15.1–45.0) | 0.004 | 0.288 | 0.001 |
| Soluble leptin receptor (ng/mL) | 23.0 (18.2–26.8) | 28.4 (23.2–31.7) | <0.001 | 22.8 (18.7–26.2) | 21.5 (19.3–24.1) | 0.444 | 0.911 | 0.000 |
| Leptin/soluble leptin receptor | 1.51 (0.88–2.92) | 0.46 (0.19–0.98) | <0.001 | 1.85 (1.18–2.67) | 1.33 (0.65–2.24) | 0.030 | 0.509 | 0.000 |
| Total adiponectin (μg/mL) | 6.6 (5.0–7.4) | 7. 0 (5.8–8.3) | 0.046 | 5.7 (4.4–6.5) | 6.9 (4.5–7.7) | 0.332 | 0.135 | 0.233 |
| HMW adiponectin (μg/mL) | 3.3 (2.1–4.5) | 3.9 (3.0–4.7) | 0.001 | 2.9 (2.1–4.1) | 3.1 (2.0–4.9) | 0.687 | 0.615 | 0.166 |
| HMW/total adiponectin (%) | 49.0 ± 14.4 | 53.5 ± 12.3 | 0.010 | 53.1 ± 14.8 | 49.9 ± 17.1 | 0.394 | 0.319 | 0.353 |
| Leptin/adiponectin | 6.26 (3.23–9.69) | 1.87 (0.91-4.09) | <0.001 | 8.79 (5.34–12.02) | 4.22 (3.49–7.87) | 0.003 | 0.104 | 0.001 |
| Glucose (mg/dL) | 84 ± 6 | 82 ± 6 | 0.087 | 83 ± 4 | 84 ± 5 | 0.255 | 0.475 | 0.190 |
| Total cholesterol (mg/dL) | 154 (144–165) | 152 (140–165) | 0.117 | 156 (145–163) | 158 (139–176) | 0.478 | 0.591 | 0.224 |
| HDL-cholesterol (mg/dL) | 49 (42–60) | 50 (41–57) | 0.763 | 48 (39–54) | 53 (43–61) | 0.190 | 0.425 | 0.439 |
| LDL-cholesterol (mg/dL) | 95 (85–99) | 89 (76–101) | 0.028 | 91 (83–103) | 95 (79–113) | 0.260 | 0.671 | 0.278 |
| Triglycerides (mg/dL) | 91 (69–115) | 75 (49–96) | 0.001 | 93 (58–135) | 82 (58–117) | 0.601 | 0.962 | 0.330 |
| Dietary intake | ||||||||
| Energy (kcal/24 h) | 1727 (1431–2136) | 1215 (962–1369) | <0.001 | 1822 (1493–2080) | 1278 (1018–1546) | <0.001 | 0.972 | 0.305 |
| Energy (% of DRI) | 93.9 (78.5–112.5) | 63.4 (49.0–73.3) | <0.001 | 82.1 (72.1–113.8) | 55.4 (41.3–78.1) | <0.001 | 0.288 | 0.716 |
| Proteins (% of energy intake) | 13.5 (12.3–15.7) | 16.8 (15.1–18.6) | <0.001 | 13.3 (12.7–15.3) | 16.1 (14.0–19.1) | 0.009 | 0.732 | 0.508 |
| Carbohydrates (% of energy intake) | 53.4 (47.7–55.9) | 51.2 (48.2–55.5) | 0.956 | 52.3 (47.8–56.1) | 51.3 (49.4–55.5) | 0.936 | 0.906 | 0.928 |
| Fat (% of energy intake) | 33.5 (31.0–38.0) | 30.9 (28.7–34.0) | 0.008 | 35.1 (31.2–37.3) | 33.3 (27.5–38.2) | 0.376 | 0.813 | 0.406 |
| Proteins (% of DRI) | 219 (170–266) | 172 (142–210) | <0.001 | 201 (160–270) | 169 (123–195) | 0.006 | 0.436 | 0.516 |
| Carbohydrates (% of DRI) | 337 (285–428) | 231 (189–287) | <0.001 | 312 (277–414) | 257 (199–300) | <0.001 | 0.870 | 0.716 |
| Fat (% of DRI) | 104 (90–136) | 71 (53–82) | <0.001 | 111 (83–144) | 70 (53–102) | 0.001 | 0.870 | 0.428 |
| LEPR Q223R A > G | LEPR K656N G > C | |||||
|---|---|---|---|---|---|---|
| Q223 (AA) (n = 22) | 223R (AG + GG) (n = 54) | p AA vs. AG + GG | K656 (GG) (n = 60) | 656N (GC + CC) (n = 16) | p GG vs. GC + CC | |
| Baseline SDS-BMI | 3.52 (3.11–4.12) | 3.32 (2.60–5.05) | 0.630 | 3.48 (2.65–4.75) | 3.40 (2.96–5.74) | 0.495 |
| Delta SDS-BMI | −0.63 ± 0.53 | −0.97 ± 0.50 | 0.010 | −0.89 ± 0.53 | −0.79 ± 0.55 | 0.494 |
| Baseline fat mass (%) | 40.2 (37.3–44.0) | 41.1 (38.9–44.3) | 0.464 | 40.7 (37.5–43.8) | 42.2 (37.9–44.2) | 0.499 |
| Delta fat mass (%) | −0.8 ± 2.9 | -3.3 ± 3.2 | 0.003 | −2.9 ± 3.4 | -1.2 ± 2.7 | 0.068 |
| Baseline fat-free mass (kg) | 29.7 ± 5.8 | 27.7 ± 7.4 | 0.114 | 28.2 ± 6.9 | 28.4 ± 7.3 | 0.819 |
| Delta fat-free mass (kg) | −0.7 ± 1.1 | −0.4 ± 1.2 | 0.402 | −0.4 ± 1.2 | -0.8 ± 1.0 | 0.342 |
| Baseline Leptin (ng/mL) | 44.0 (28.3–65.9) | 37.0 (22.8–54.6) | 0.166 | 37.0 (21.5–55.1) | 46.0 (32.4–61.4) | 0.125 |
| Delta Leptin (ng/mL) | −26.6 ± 24.4 | −21.2 ± 20.5 | 0.334 | −22.2 ± 21.4 | −24.9 ± 23.2 | 0.670 |
| Baseline sOB-R (ng/mL) | 22.0 (17.6–27.6) | 23.1 (19.6–26.6) | 0.639 | 23.1 (18.2–26.5) | 22.3 (19.3–28.3) | 0.828 |
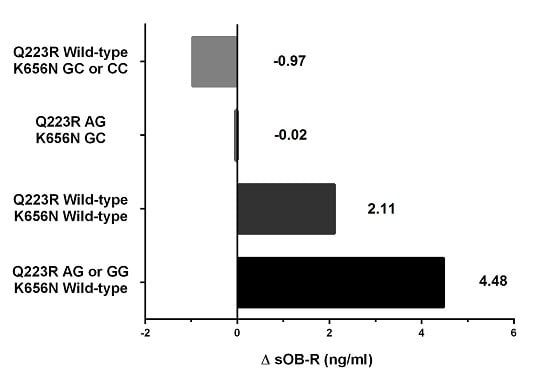
| Delta sOB-R (ng/mL) | 1.2 ± 6.3 | 3.9 ± 6.7 | 0.108 | 4.0 ± 6.4 | 0.0 ± 6.9 | 0.032 |
| Baseline Leptin/sOB-R | 2.24 (1.09–3.29) | 1.50 (0.89–2.50) | 0.204 | 1.42 (.86–2.69) | 2.27 (1.43–3.02) | 0.198 |
| Delta Leptin/sOB-R | −0.82 (−2.52–−0.15) | −0.85 (−1.68–−0.36) | 0.891 | −0.82 (-1.94–−0.34) | −1.02 (−2.07–−0.09) | 0.674 |
| Baseline Leptin/adiponectin | 7.79 (5.37–11.86) | 6.05 (3.32–9.98) | 0.248 | 6.63 (3.34–10.63) | 7.06 (5.13–10.98) | 0.333 |
| Delta Leptin/adiponectin | −4.5 ± 3.7 | −4.0 ± 3.8 | 0.628 | −4.22 ± 3.62 | −3.72 ± 4.36 | 0.678 |
| Q223R/K656N Genotype | Change in Fat Mass (%) | Change in Fat-Free Mass (kg) | ||
|---|---|---|---|---|
| Pearson r | p | Pearson r | p | |
| Wild-type/Wild-type | 0.858 | <0.001 | 0.334 | 0.244 |
| AG or GG/Wild-type | 0.519 | <0.001 | 0.194 | 0.197 |
| Wild-type/GC or CC | 0.372 | 0.364 | 0.904 | 0.002 |
| AG/GC | 0.566 | 0.144 | 0.683 | 0.062 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, J.; Kuryłowicz, A.; Mierzejewska, E.; Ambroszkiewicz, J.; Chełchowska, M.; Weker, H.; Puzianowska-Kuźnicka, M. Complementary Effects of Genetic Variations in LEPR on Body Composition and Soluble Leptin Receptor Concentration after 3-Month Lifestyle Intervention in Prepubertal Obese Children. Nutrients 2016, 8, 328. https://doi.org/10.3390/nu8060328
Gajewska J, Kuryłowicz A, Mierzejewska E, Ambroszkiewicz J, Chełchowska M, Weker H, Puzianowska-Kuźnicka M. Complementary Effects of Genetic Variations in LEPR on Body Composition and Soluble Leptin Receptor Concentration after 3-Month Lifestyle Intervention in Prepubertal Obese Children. Nutrients. 2016; 8(6):328. https://doi.org/10.3390/nu8060328
Chicago/Turabian StyleGajewska, Joanna, Alina Kuryłowicz, Ewa Mierzejewska, Jadwiga Ambroszkiewicz, Magdalena Chełchowska, Halina Weker, and Monika Puzianowska-Kuźnicka. 2016. "Complementary Effects of Genetic Variations in LEPR on Body Composition and Soluble Leptin Receptor Concentration after 3-Month Lifestyle Intervention in Prepubertal Obese Children" Nutrients 8, no. 6: 328. https://doi.org/10.3390/nu8060328
APA StyleGajewska, J., Kuryłowicz, A., Mierzejewska, E., Ambroszkiewicz, J., Chełchowska, M., Weker, H., & Puzianowska-Kuźnicka, M. (2016). Complementary Effects of Genetic Variations in LEPR on Body Composition and Soluble Leptin Receptor Concentration after 3-Month Lifestyle Intervention in Prepubertal Obese Children. Nutrients, 8(6), 328. https://doi.org/10.3390/nu8060328