Efficacy of Folic Acid Supplementation in Autistic Children Participating in Structured Teaching: An Open-Label Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Nutritional Supplements
2.3. Structured Teaching
2.4. Evaluation
2.5. Sample Treatment and Metabolite Analysis
2.6. Statistics
3. Results
3.1. Clinical Features
3.2. Behavior Change over the Intervention Period
3.3. Metabolic Profile
4. Discussion
4.1. Behavioral Improvements with Intervention
4.2. Metabolic Profile before and after Intervention
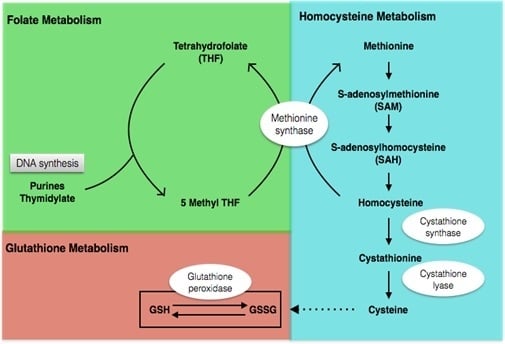
4.2.1. Metabolic Profile before Intervention
4.2.2. Metabolic Profile after Intervention
4.3. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AB | adaptive behaviors |
| ABC | Autism Behavior Checklist |
| AE | affective expression |
| ASD | Autism Spectrum Disorder |
| ATEC | Autism Treatment Evaluation Checklist |
| CARS | Children Autism Rating Scale |
| CBS | cystathionine-beta-synthase |
| CMB | characteristic motor behaviors |
| CVB | characteristic verbal behaviors |
| CVP | cognitive verbal/preverbal |
| DHFR | dihydrofolate reductase |
| EL | expressive language |
| FM | fine motor |
| GM | gross motor |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione disulfide |
| Hcy | homocysteine |
| PB | problem behaviors |
| PEP-3 | Psychoeducational Profile-third edition |
| PSC | personal self-care |
| RL | receptive language |
| SAM | S-adenosylmethionine |
| SR | social reciprocity |
| TEACCH | The Treatment and Education of Autistic and Related Communication Handicapped Children |
| VMI | visual motor imitation |
References
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Blumberg, S.J. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National health interview survey. Natl. Health Stat. Report. 2015, 13, 1–20. [Google Scholar]
- Main, P.A.; Angley, M.T.; O’Doherty, C.E.; Thomas, P.; Fenech, M. The potential role of the antioxidant and detoxification properties of glutathione in autism spectrum disorders: A systematic review and meta-analysis. Nutr. Metab. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Waly, M.I.; Al-Farsi, Y.M.; Essa, M.M.; Al-Sharbati, M.M.; Deth, R.C. Hyperhomocysteinemia among Omani autistic children: A case-control study. Acta Biochim. Pol. 2011, 58, 547–551. [Google Scholar] [PubMed]
- Al-Farsi, Y.M.; Waly, M.I.; Deth, R.C.; Al-Sharbati, M.M.; Al-Shafaee, M.; Al-Farsi, O.; Al-Khaduri, M.M.; Gupta, I.; Ali, A.; Al-Khalili, M.; et al. Low folate and vitamin B12 nourishment is common in Omani children with newly diagnosed autism. Nutrition 2013, 29, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Cosar, A.; Ipcioglu, O.M.; Ozcan, O.; Gultepe, M. Folate and homocysteine metabolisms and their roles in the biochemical basis of neuropsychiatry. Turk. J. Med. Sci. 2014, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fulceri, F.; Morelli, M.; Santocchi, E.; Cena, H.; Del Bianco, T.; Narzisi, A.; Calderoni, S.; Muratori, F. Gastrointestinal symptoms and behavioral problems in preschoolers with Autism Spectrum Disorder. Dig. Liver Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Audhya, T.; Chauhan, V. Brain region-specific glutathione redox imbalance in autism. Neurochem. Res. 2012, 37, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W.; Walters, L.; James, S.J. Effectiveness of methylcobalamin and folinic acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res. Treat. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kvestad, I.; Taneja, S.; Kumar, T.; Hysing, M.; Refsum, H.; Yajnik, C.S.; Bhandari, N.; Strand, T.A. Vitamin B12 and folic acid improve gross motor and problem-solving skills in young north Indian children: A randomized placebo-controlled trial. PLoS ONE 2015, 10, e0129915. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (Childhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Gracely, E.J.; Lee, B.K. Serum folate but not vitamin B-12 concentrations are positively associated with cognitive test scores in children aged 6–16 years. J. Nutr. 2013, 143, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Virues-Ortega, J.; Julio, F.M.; Pastor-Barriuso, R. The TEACCH program for children and adults with autism: A meta-analysis of intervention studies. Clin. Psychol. Rev. 2013, 33, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, J. Factor structure of the childhood autism rating scale as per DSM-5. Pediatr. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Magiati, I.; Moss, J.; Yates, R.; Charman, T.; Howlin, P. Is the Autism Treatment Evaluation Checklist a useful tool for monitoring progress in children with autism spectrum disorders? J. Intellect. Disabil. Res. 2011, 55, 302–312. [Google Scholar] [CrossRef] [PubMed]
- De Giacomo, A.; Craig, F.; Cristella, A.; Terenzio, V.; Buttiglione, M.; Margari, L. Can PEP-3 provide a cognitive profile in children with ASD? A comparison between the developmental ages of pep-3 and IQ of leiter-R. J. Appl. Res. Intellect. Disabil. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Chiang, F.M.; Tseng, M.H.; Fu, C.P.; Hsieh, C.L. Responsiveness of the psychoeducational profile-third edition for children with autism spectrum disorders. J. Autism Dev. Disord. 2011, 41, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Valeri, G.; Sonnino, F.; Fontana, I.; Mammone, A.; Vicari, S. A longitudinal study of the teacch program in different settings: the potential benefits of low intensity intervention in preschool children with autism spectrum disorder. J. Autism Dev. Disord. 2014, 44, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Sahoo, T.; Hyland, K.; Bottiglieri, T.; Peters, S.; del Gaudio, D.; Roa, B.; Curry, S.; Zhu, H.; Finnell, R.H.; et al. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology 2005, 64, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Schaevitz, L.; Berger-Sweeney, J.; Ricceri, L. One-carbon metabolism in neurodevelopmental disorders: Using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neurosci. Biobehav. Rev. 2014, 46, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Puig-Alcaraz, C.; Fuentes-Albero, M.; Calderon, J.; Garrote, D.; Cauli, O. Increased homocysteine levels correlate with the communication deficit in children with autism spectrum disorder. Psychiatry Res. 2015, 229, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ayesa-Arriola, R.; Perez-Iglesias, R.; Rodriguez-Sanchez, J.M.; Mata, I.; Gomez-Ruiz, E.; Garcia-Unzueta, M.; Martinez-Garcia, O.; Tabares-Seisdedos, R.; Vazquez-Barquero, J.L.; Crespo-Facorro, B. Homocysteine and cognition in first-episode psychosis patients. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Groger, A.; Kolb, R.; Schafer, R.; Klose, U. Dopamine reduction in the substantia Nigra of Parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS ONE 2014, 9, e84081. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Barone, C.; Romano, C.; Salluzzo, M.G.; Giambirtone, M.; Morale, M.C.; Calogero, A.E.; Grillo, L.; Bosco, P. A peculiar VNTR in the cystathionine beta-synthase gene is a risk factor for Down Syndrome. Cell Mol. Biol. (Noisy-le-grand) 2015, 61, 49–51. [Google Scholar]
- Kamat, P.K.; Vacek, J.C.; Kalani, A.; Tyagi, N. Homocysteine induced cerebrovascular dysfunction: A link to Alzheimer’s disease etiology. Open Neurol. J. 2015, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Morisaki, N.; Honda, Y.; Sampei, M.; Tani, Y. Chemicals, nutrition, and autism spectrum disorder: A mini-review. Front. Neurosci. 2016, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.P.; Dronca, E.; Kaucsar, T.; Craciun, E.C.; Endreffy, E.; Ferencz, B.K.; Iftene, F.; Benga, I.; Cornean, R.; Banerjee, R.; et al. One carbon metabolism disturbances and the C677T MTHFR gene polymorphism in children with autism spectrum disorders. J. Cell Mol. Med. 2009, 13, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D.; De Santo, N.G. Homocysteine and oxidative stress. Amino Acids 2003, 25, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kaluzna-Czaplinska, J.; Michalska, M.; Rynkowski, J. Homocysteine level in urine of autistic and healthy children. Acta Biochim. Pol. 2011, 58, 31–34. [Google Scholar] [PubMed]
- Tu, W.J.; Chen, H.; He, J. Application of LC-MS/MS analysis of plasma amino acids profiles in children with autism. J. Clin. Biochem. Nutr. 2012, 51, 248–249. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [PubMed]
- Pecorelli, A.; Leoncini, S.; De Felice, C.; Signorini, C.; Cerrone, C.; Valacchi, G.; Ciccoli, L.; Hayek, J. Non-protein-bound iron and 4-hydroxynonenal protein adducts in classic autism. Brain Dev. 2013, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ilango, K.; Singh, P.K.; Karmakar, D.; Singh, G.P.; Kumari, R.; Dubey, G.P. Age dependent levels of plasma homocysteine and cognitive performance. Behav. Brain Res. 2015, 283, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hoey, L.; McNulty, H.; Askin, N.; Dunne, A.; Ward, M.; Pentieva, K.; Strain, J.; Molloy, A.M.; Flynn, C.A.; Scott, J.M. Effect of a voluntary food fortification policy on folate, related B vitamin status, and homocysteine in healthy adults. Am. J. Clin. Nutr. 2007, 86, 1405–1413. [Google Scholar] [PubMed]
- Frye, R.E.; Delatorre, R.; Taylor, H.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S.J. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl. Psychiatry 2013, 3, e273. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A. Increased glutamate and homocysteine and decreased glutamine levels in autism: A review and strategies for future studies of amino acids in autism. Dis. Markers 2013, 35, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Kirsch, S.H.; Dilmann, S.; Klein, C.; Eckert, R.; Geisel, J.; Herrmann, W. Folic acid causes higher prevalence of detectable unmetabolized folic acid in serum than B-complex: A randomized trial. Eur. J. Nutr. 2016, 55, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Molloy, A.M.; Mills, J.L.; Fan, R.; Wang, Y.; Gibney, E.R.; Shane, B.; Brody, L.C.; Parle-McDermott, A. The dihydrofolate reductase 19 bp polymorphism is not associated with biomarkers of folate status in healthy young adults, irrespective of folic acid intake. J. Nutr. 2015, 145, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Pentieva, K.; Selhub, J.; Paul, L.; Molloy, A.M.; McNulty, B.; Ward, M.; Marshall, B.; Dornan, J.; Reilly, R.; Parle-McDermott, A.; et al. Evidence from a randomized trial that exposure to supplemental folic acid at recommended levels during pregnancy does not lead to increased unmetabolized folic acid concentrations in maternal or cord blood. J. Nutr. 2016, 146, 494–500. [Google Scholar] [CrossRef] [PubMed]



| Features | Intervention (n = 44) | Control (n = 22) |
|---|---|---|
| Age (month) | 57.23 ± 15.06 | 51.75 ± 12.72 |
| Sex (male:female) | 37:7 | 17:5 |
| ABC | 54.55 ± 26.58 | 67.59 ± 27.60 |
| CARS | 33.86 ± 7.08 | 33.41 ± 6.04 |
| ATEC | ||
| Communication | 9.93 ± 7.02 | 11.09 ± 6.91 |
| Sociability | 8.93 ± 5.84 | 10.09 ± 5.30 |
| Sensory and cognitive awareness | 12.86 ± 7.19 | 15.14 ± 7.03 |
| Health behavioral problem | 16.95 ± 7.69 | 21.05 ± 9.81 |
| Total | 48.68 ± 21.43 | 57.36 ± 20.38 |
| PEP-3 | ||
| CVP | 12.00 ± 3.00 | 12.95 ± 2.38 |
| EL | 11.41 ± 3.41 | 11.91 ± 2.86 |
| RL | 12.36 ± 2.42 | 13.32 ± 1.96 |
| FM | 11.80 ± 1.79 | 11.91 ± 2.00 |
| GM | 12.07 ± 1.78 | 12.36 ± 1.92 |
| VMI | 11.55 ± 2.17 | 12.00 ± 2.16 |
| AE | 11.80 ± 2.06 | 10.95 ± 2.30 |
| SR | 10.36 ± 2.08 | 10.50 ± 1.87 |
| CMB | 11.98 ± 2.39 | 11.95 ± 2.30 |
| CVB | 8.00 ± 3.58 | 9.05 ± 2.90 |
| Communication | 35.77 ± 8.21 | 38.18 ± 6.71 |
| Motor | 35.41 ± 5.18 | 36.27 ± 5.54 |
| Maladaptive behavior | 42.14 ± 7.84 | 42.45 ± 7.31 |
| PB | 8.25 ± 2.36 | 8.27 ± 2.39 |
| PSC | 11.70 ± 2.41 | 12.09 ± 2.27 |
| AB | 11.14 ± 2.36 | 11.36 ± 2.63 |
| Scales | Point | Intervention (n = 44) | Control (n = 22) | p 1 | p 2 | p 3 |
|---|---|---|---|---|---|---|
| ABC | Baseline | 54.55 ± 26.58 | 67.59 ± 27.60 | 0.424 | <0.001 * | 0.080 |
| Endpoint | 39.40 ± 26.73 | 46.18 ± 22.71 | ||||
| CARS | Baseline | 33.86 ± 7.08 | 33.41 ± 6.04 | 0.331 | 0.001 * | 0.684 |
| Endpoint | 29.34 ± 5.52 | 30.82 ± 5.06 | ||||
| ATEC | ||||||
| communication | Baseline | 9.93 ± 7.02 | 11.09 ± 6.91 | 0.583 | <0.001 * | 0.354 |
| Endpoint | 6.84 ± 5.65 | 8.64 ± 6.29 | ||||
| sociability | Baseline | 8.93 ± 5.84 | 10.09 ± 5.30 | 0.024 * | 0.002 | 0.020 |
| Endpoint | 5.23 ± 3.72 | 9.45 ± 5.12 | ||||
| sensory and cognitive awareness | Baseline | 12.86 ± 7.19 | 15.14 ± 7.03 | 0.349 | <0.001 * | 0.321 |
| Endpoint | 11.36 ± 7.15 | 12.50 ± 5.71 | ||||
| health behavioral problem | Baseline | 16.95 ± 7.69 | 21.05 ± 9.81 | 0.542 | <0.001 * | 0.059 |
| Endpoint | 12.86 ± 7.00 | 15.77 ± 7.79 | ||||
| total | Baseline | 48.68 ± 21.43 | 57.36 ± 20.38 | 0.719 | <0.001 * | 0.052 |
| Endpoint | 36.30 ± 17.49 | 46.36 ± 18.56 | ||||
| PEP-3 | ||||||
| CVP | Baseline | 12.00 ± 3.00 | 12.95 ± 2.38 | 0.020 * | <0.001 | 0.593 |
| Endpoint | 14.20 ± 1.97 | 13.91 ± 2.79 | ||||
| EL | Baseline | 11.41 ± 3.41 | 11.91 ± 2.86 | 0.451 | <0.001 * | 0.715 |
| Endpoint | 13.07 ± 3.19 | 13.14 ± 2.87 | ||||
| RL | Baseline | 12.36 ± 2.42 | 13.32 ± 1.96 | 0.027 * | <0.001 | 0.313 |
| Endpoint | 13.75 ± 1.40 | 13.73 ± 1.75 | ||||
| FM | Baseline | 11.80 ± 1.79 | 11.91 ± 2.00 | 0.328 | <0.001 * | 0.447 |
| Endpoint | 12.48 ± 1.68 | 13.00 ± 1.75 | ||||
| GM | Baseline | 12.07 ± 1.78 | 12.36 ± 1.92 | 0.954 | 0.001 * | 0.954 |
| Endpoint | 12.77 ± 0.99 | 13.09 ± 1.31 | ||||
| VMI | Baseline | 11.55 ± 2.17 | 12.00 ± 2.16 | 0.860 | 0.010 * | 0.349 |
| Endpoint | 12.27 ± 1.48 | 13.64 ± 1.97 | ||||
| AE | Baseline | 11.80 ± 2.06 | 10.95 ± 2.30 | 0.047 * | 0.012 | 0.674 |
| Endpoint | 11.98 ± 2.25 | 12.45 ± 1.26 | ||||
| SR | Baseline | 10.36 ± 2.08 | 10.50 ± 1.87 | 0.717 | <0.001 * | 0.586 |
| Endpoint | 11.30 ± 2.09 | 11.64 ± 1.65 | ||||
| CMB | Baseline | 11.98 ± 2.39 | 11.95 ± 2.30 | 0.149 | 0.628 | 0.404 |
| Endpoint | 11.66 ± 2.58 | 12.59 ± 2.26 | ||||
| CVB | Baseline | 8.00 ± 3.58 | 9.05 ± 2.90 | 0.837 | 0.001 * | 0.218 |
| Endpoint | 9.64 ± 3.72 | 10.50 ± 2.65 | ||||
| Communication | Baseline | 35.77 ± 8.21 | 38.18 ± 6.71 | 0.037 * | <0.001 | 0.536 |
| Endpoint | 41.02 ± 5.91 | 40.77 ± 7.04 | ||||
| Motor | Baseline | 35.41 ± 5.18 | 36.27 ± 5.54 | 0.749 | <0.001 * | 0.359 |
| Endpoint | 37.52 ± 3.79 | 38.73 ± 4.69 | ||||
| Maladaptive behaviors | Baseline | 42.14 ± 7.84 | 42.45 ± 7.31 | 0.285 | 0.001 * | 0.433 |
| Endpoint | 44.57 ± 9.59 | 47.18 ± 6.53 | ||||
| PB | Baseline | 8.25 ± 2.36 | 8.27 ± 2.39 | 0.936 | 0.076 | 0.983 |
| Endpoint | 9.05 ± 2.90 | 9.00 ± 2.65 | ||||
| PSC | Baseline | 11.70 ± 2.41 | 12.09 ± 2.27 | 0.612 | 0.131 | 0.636 |
| Endpoint | 12.34 ± 1.70 | 12.41 ± 2.48 | ||||
| AB | Baseline | 11.14 ± 2.36 | 11.36 ± 2.63 | 0.202 | 0.010 * | 0.786 |
| Endpoint | 12.32 ± 2.59 | 11.77 ± 2.51 |
| Metabolite Levels | Typical Group (n = 29) | Autistic Group (n = 29) | p Value * | |
|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | |||
| VitB12 (pmol/mL) | 523.30 ± 186.51 | 555.24 ± 249.00 | 544.17 ± 213.57 | 0.723 |
| Folic acid (nmol/L) | 28.60 ± 8.67 | 21.60 ± 9.09 a | 64.5 ± 14.45 a | <0.001 * |
| Hcy (µmol/L) | 7.80 ± 1.13 | 8.80 ± 2.29 a | 5.65 ± 1.39 a | <0.001 * |
| tGSH (µmol/L) | 17.84 ± 5.03 | 13.37 ± 4.26 a | 20.07 ± 4.03 | <0.001 * |
| GSSG (µmol/L) | 5.79 ± 2.81 | 9.44 ± 4.45 a | 6.68 ± 2.68 | 0.009 * |
| tGSH/GSSG | 4.26 ± 3.25 | 1.61 ± 0.59 a | 3.67 ± 2.01 | <0.001 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Zou, M.; Zhao, D.; Xia, W.; Wu, L. Efficacy of Folic Acid Supplementation in Autistic Children Participating in Structured Teaching: An Open-Label Trial. Nutrients 2016, 8, 337. https://doi.org/10.3390/nu8060337
Sun C, Zou M, Zhao D, Xia W, Wu L. Efficacy of Folic Acid Supplementation in Autistic Children Participating in Structured Teaching: An Open-Label Trial. Nutrients. 2016; 8(6):337. https://doi.org/10.3390/nu8060337
Chicago/Turabian StyleSun, Caihong, Mingyang Zou, Dong Zhao, Wei Xia, and Lijie Wu. 2016. "Efficacy of Folic Acid Supplementation in Autistic Children Participating in Structured Teaching: An Open-Label Trial" Nutrients 8, no. 6: 337. https://doi.org/10.3390/nu8060337
APA StyleSun, C., Zou, M., Zhao, D., Xia, W., & Wu, L. (2016). Efficacy of Folic Acid Supplementation in Autistic Children Participating in Structured Teaching: An Open-Label Trial. Nutrients, 8(6), 337. https://doi.org/10.3390/nu8060337