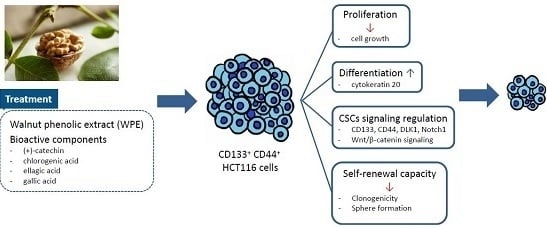
Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of WPE
2.2. Analysis of Phenolic Bioactive Compounds by High-Pressure Liquid Chromatography (HPLC)
2.3. Cell Culture
2.4. Human Primary Cell Isolation
2.5. Isolation of CSCs
2.6. Cell Proliferation Assay
2.7. RNA Preparation and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.8. Western Blot Analysis
2.9. Colony Formation Assay
2.10. Sphere Formation Assay
2.11. Statistical Analyses
3. Results
3.1. Phenolic Compounds Detected in WPE by HPLC
3.2. WPE and Its Bioactive Compounds Suppress the Cell Proliferation of Colon CSCs
3.3. WPE and Its Bioactive Compounds Induce the Cell Differentiation of Colon CSCs
3.4. WPE and Its Bioactive Compounds Suppress Colon CSCs Markers, Including CD133, CD44, DLK1, and Notch1 as Well as Wnt/β-Catenin Signaling in Colon CSCs
3.5. WPE and Its Bioactive Compounds Suppress the Self-Renewal Capacity of Colon CSCs
3.6. WPE Down-Regulates CD133, CD44, DLK1, and Notch1 in Human Primary Cells Obtained from CRC Tissue
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: Aacr workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Francipane, M.G.; Medema, J.P.; Stassi, G. Colon cancer stem cells: Promise of targeted therapy. Gastroenterology 2010, 138, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sul, H.S. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009, 9, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Forman, S.J.; Bhatia, R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene 2005, 24, 4472–4476. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Karsan, A. Recent insights into the role of notch signaling in tumorigenesis. Blood 2006, 107, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [PubMed]
- Dreher, M.L.; Maher, C.V.; Kearney, P. The traditional and emerging role of nuts in healthful diets. Nutr. Rev. 1996, 54, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Khanduja, K.L.; Gandhi, R.K.; Kapur, S.; Sharma, R.R. Influence of ellagic acid on antioxidant defense system and lipid peroxidation in mice. Biochem. Pharmacol. 1991, 42, 1441–1445. [Google Scholar] [CrossRef]
- Constantinou, A.; Stoner, G.D.; Mehta, R.; Rao, K.; Runyan, C.; Moon, R. The dietary anticancer agent ellagic acid is a potent inhibitor of DNA topoisomerases in vitro. Nutr. Cancer 1995, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.B. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J. Nutr. 2002, 132, 1062s–1101s. [Google Scholar] [PubMed]
- Jenab, M.; Ferrari, P.; Slimani, N.; Norat, T.; Casagrande, C.; Overad, K.; Olsen, A.; Stripp, C.; Tjonneland, A.; Boutron-Ruault, M.C.; et al. Association of nut and seed intake with colorectal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1595–1603. [Google Scholar]
- Yeh, C.C.; You, S.L.; Chen, C.J.; Sung, F.C. Peanut consumption and reduced risk of colorectal cancer in women: A prospective study in Taiwan. World J. Gastroenterol. 2006, 12, 222–227. [Google Scholar] [PubMed]
- Hardman, W.E.; Ion, G.; Akinsete, J.A.; Witte, T.R. Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse. Nutr. Cancer 2011, 63, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Ion, G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr. Cancer 2008, 60, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J.; Blackburn, G.; Mantzoros, C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition (Burbank, Los Angeles County, Calif.) 2012, 28, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, Y.-S.; Lee, J.; Lee, J.H.; Choi, S.-W.; Kim, Y. Compositional analysis of walnut lipid extracts and properties as an anti-cancer stem cell regulator via suppression of the self-renewal capacity. Food Sci. Biotechnol. 2016, 25, 623–629. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Zhu, H.; Young, J.C. Polyphenolic profiles and antioxidant activities of heartnut (Juglans ailanthifolia Var. cordiformis) and Persian walnut (Juglans regia L.). J. Agric. Food Chem. 2006, 54, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jeronimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M.P. Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm. Biol. 2011, 49, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Min, S.J.; Lim, J.Y.; Kim, H.R.; Kim, S.J.; Kim, Y. Sasa quelpaertensis leaf extract inhibits colon cancer by regulating cancer cell stemness in vitro and in vivo. Int. J. Mol. Sci. 2015, 16, 9976–9997. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Kim, Y. Mulberry leaf extract inhibits cancer cell stemness in neuroblastoma. Nutr. Cancer 2012, 64, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, Y.S.; Kim, K.M.; Min, S.J.; Kim, Y. Beta-carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness. Biochem. Biophys. Res. Commun. 2014, 450, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lin, Q.; Zelterman, D.; Yun, Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009, 69, 9271–9280. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z. Seer Cancer Statistics Review, 1975–2011; National Cancer Institute: Bethesda, MD, USA, 2014.
- Jiang, W.; Peng, J.; Zhang, Y.; Cho, W.; Jin, K. The implications of cancer stem cells for cancer therapy. Int. J. Mol. Sci. 2012, 13, 16636–16657. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Kim, L.G.; Kong, H.H.; Low, L.Y.; Iacopetta, B.; Soong, R.; Salto-Tellez, M. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod. Pathol. 2010, 23, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Horst, D.; Scheel, S.K.; Liebmann, S.; Neumann, J.; Maatz, S.; Kirchner, T.; Jung, A. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J. Pathol. 2009, 219, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.; Purnell, P.; Nguyen, L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp. Mol. Pathol. 2009, 86, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Chiou, S.H.; Su, Y. Maintenance of the stemness in CD44(+) HCT-15 and HCT-116 human colon cancer cells requires mir-203 suppression. Stem Cell Res. 2014, 12, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Ohkuma, M.; Sakashita, H.; Matsuzaki, S.; Tanaka, F.; Mimori, K.; Kamohara, Y.; Inoue, H.; Mori, M. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann. Surg. Oncol. 2008, 15, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Pan, F.; Jiang, H.; Chen, J.F.; Pei, L.; Xie, F.W.; Liang, H.J. Highly enriched CD133(+)CD44(+) stem-like cells with CD133(+)CD44(high) metastatic subset in HCT116 colon cancer cells. Clin. Exp. Metastasis 2011, 28, 751–763. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, F.; Witte, T.R.; Hardman, W.E.; Claudio, P.P. Omega-3 eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy. PLoS ONE 2013, 8, e69760. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A.; Ibeh, C.; Han, L.; Desbordes, C. Inhibition of growth and induction of differentiation markers by polyphenolic molecules and histone deacetylase inhibitors in colon cancer cells. Anticancer Res. 2010, 30, 311–318. [Google Scholar] [PubMed]
- Li, L.; Tan, J.; Zhang, Y.; Han, N.; Di, X.; Xiao, T.; Cheng, S.; Gao, Y.; Liu, Y. DLK1 promotes lung cancer cell invasion through upregulation of MMP9 expression depending on notch signaling. PLoS ONE 2014, 9, e91509. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (−)-epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male c57bl/ksj-db/db mice. Cancer Prev. Res. (Phila) 2008, 1, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.K.; Yang, C.H. Quercetin, a potent inhibitor against beta-catenin/tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Nagi, A.H. Paraquat and adrenal cortical necrosis. Br. Med. J. 1970, 2, 669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Zou, C.L.; Duan, Y.X.; Wu, F.; Li, G. Activity guided isolation and modification of juglone from Juglans regia as potent cytotoxic agent against lung cancer cell lines. BMC Complement. Altern. Med. 2015, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Sugie, S.; Okamoto, K.; Rahman, K.M.; Tanaka, T.; Kawai, K.; Yamahara, J.; Mori, H. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998, 127, 177–183. [Google Scholar] [CrossRef]
- Chiang, E.P.; Tsai, S.Y.; Kuo, Y.H.; Pai, M.H.; Chiu, H.L.; Rodriguez, R.L.; Tang, F.Y. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and Ampk signaling pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kakuda, Y.; Yeung, D. Antioxidative properties of lycopene and other carotenoids from tomatoes: Synergistic effects. Biofactors 2004, 21, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, W.C.; Solis, D.Y.; Hebrant, A.; Detours, V.; Dumont, J.E.; Maenhaut, C. Human cancer cell lines: Experimental models for cancer cells in situ? For cancer stem cells? Biochim. Biophys. Acta 2009, 1795, 92–103. [Google Scholar] [CrossRef] [PubMed]






| Target | Forward Primer (5′ →3′) | Reverse Primer (3′ →5′) |
|---|---|---|
| CD133 | TGGATGCAGAACTTGACAACGT | ATACCTGCTACGACAGTCGTGGT |
| CD44 | CCAATGCCTTTGATGGACC | TCTGTCTGTGCTGTCGGTGAT |
| DLK1 | CTGAAGGTGTCCATGAAAGAG | GCTGAAGGTGGTCATGTCGAT |
| Notch1 | GAGGCGTGGCAGACTATGC | CTTGTACTCCGTCAGCGTGA |
| β-actin | ATTGGCAATGAGCGGTTC | GGATGCCACAGGACTCCAT |
| GAPDH | AGAAGGCTGGGGCTCATTTG | AGGGGCCATCCACAGTCTTC |
| Phenolic Compounds | Concentration (mg/100 g of WPE) |
|---|---|
| Gallic acid | 10.7 |
| (+)-Catechin | 137.5 |
| Chlorogenic acid | 13.6 |
| Ellagic acid | 12.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, Y.-S.; Lee, J.; Heo, S.C.; Lee, K.L.; Choi, S.-W.; Kim, Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients 2016, 8, 439. https://doi.org/10.3390/nu8070439
Lee J, Kim Y-S, Lee J, Heo SC, Lee KL, Choi S-W, Kim Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients. 2016; 8(7):439. https://doi.org/10.3390/nu8070439
Chicago/Turabian StyleLee, Jisoo, Yoo-Sun Kim, JaeHwan Lee, Seung Chul Heo, Kook Lae Lee, Sang-Woon Choi, and Yuri Kim. 2016. "Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness" Nutrients 8, no. 7: 439. https://doi.org/10.3390/nu8070439
APA StyleLee, J., Kim, Y. -S., Lee, J., Heo, S. C., Lee, K. L., Choi, S. -W., & Kim, Y. (2016). Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients, 8(7), 439. https://doi.org/10.3390/nu8070439