Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Kits
2.2. Sample Preparation
2.2.1. Separation and Purification of Ginsenoside Rg5
2.2.2. HPLC Analysis of Ginsenoside Rg5
2.3. Animals and Experimental Protocol
2.4. Assessment of Biochemical Parameters
2.5. Measurement of Kidney TNF-α and IL-1β Levels
2.6. Histopathological Analysis
2.7. Hoechst 33258 Staining
2.8. Immunohistochemistry (IHC) and Immunofluorescence
2.9. TUNEL Assay
2.10. Western Blotting Analysis
2.11. Statistical Analysis
3. Results
3.1. Isolation and Identification of Ginsenoside Rg5
3.2. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Renal Dysfunction
3.3. Ginsenoside Rg5 Attenuates Cisplatin-Induced Oxidative Stress
3.4. Ginsenoside Rg5 Attenuates Cisplatin-Induced Renal Inflammation
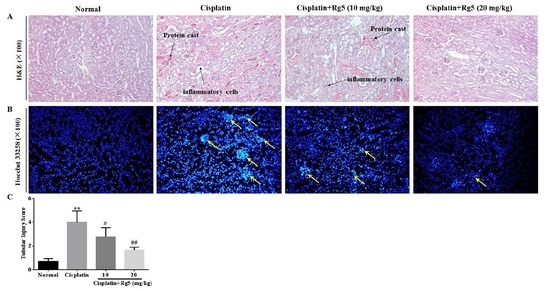
3.5. Ginsenoside Rg5 Attenuates Cisplatin-Induced Renal Histopathological Changes
3.6. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Tubular Apoptosis


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| SOD | Superoxide dismutase |
| BUN | Blood urea nitrogen |
| MDA | Malondialdehyde |
| CRE | Creatinine |
| IL-1β | Interleukin-1β |
| GSH | Glutathione |
| TNF-α | Tumor necrosis factor-α |
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dang, C.; Kang, H.; Dai, Z.; Lin, S.; Guan, H.; Liu, X.; Wang, X.; Hui, W. Saikosaponin-d reduces cisplatin-induced nephrotoxicity by repressing ros-mediated activation of mapk and nf-kappab signalling pathways. Int. Immunopharmacol. 2015, 28, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hamad, R.; Jayakumar, C.; Ranganathan, P.; Mohamed, R.; El-Hamamy, M.M.; Dessouki, A.A.; Ibrahim, A.; Ramesh, G. Honey feeding protects kidney against cisplatin nephrotoxicity through suppression of inflammation. Clin. Exp. Pharmacol. Physiol. 2015, 42, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chen, J.; Shen, K.; Wang, X.; Wang, P.; Fu, G.; Meng, H.; Wang, Y.; Jin, B. Mitochondrial modulation by epigallocatechin 3-gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and nf-kb in mice. PLoS ONE 2015, 10, e0124775. [Google Scholar] [CrossRef] [PubMed]
- Song, K.I.; Park, J.Y.; Lee, S.; Lee, D.; Jang, H.J.; Kim, S.N.; Ko, H.; Kim, H.Y.; Lee, J.W.; Hwang, G.S.; et al. Protective effect of tetrahydrocurcumin against cisplatin-induced renal damage: In vitro and in vivo studies. Planta Med. 2015, 81, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Bunel, V.; Antoine, M.H.; Nortier, J.; Duez, P.; Stevigny, C. In vitro effects of Panax ginseng in aristolochic acid-mediated renal tubulotoxicity: Apoptosis versus regeneration. Planta Med. 2015, 81, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Yu, M.; Kim, M.; Choi, H.S.; Kang, D.H. Renoprotective effect of red ginseng in gentamicin-induced acute kidney injury. Lab. Investig. 2014, 94, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, M.Y.; Son, H.Y.; Park, B.K.; Ryu, S.Y.; Jung, J.Y. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014, 80, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Meng, Q.T.; Jiang, Y.; Liu, H.M.; Lei, S.Q.; Su, W.T.; Duan, W.N.; Wu, Y.; Xia, Z.Y.; Xia, Z.Y. Protective effect of ginsenoside Rb1 against intestinal ischemia-reperfusion induced acute renal injury in mice. PLoS ONE 2013, 8, e80859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokozawa, T.; Liu, Z.W. The role of ginsenoside-rd in cisplatin-induced acute renal failure. Ren. Fail. 2000, 22, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Gu, J.; Chen, L.; Hou, W.; Jin, Y.; Wang, Y. Bioconversion of ginsenoside rd to ginsenoside m1 by snailase hydrolysis and its enhancement effect on insulin secretion in vitro. Die Pharm. 2015, 70, 340–346. [Google Scholar]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.J.; Zhao, L.C.; Zheng, Y.N.; Chen, L.; Yang, G.L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound k on type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.J.; Jeon, J.N.; Wang, C.; Min, J.W.; Noh, H.Y.; Yang, D.C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, X.; Wang, M.; Li, B.; Liu, Z.; Liu, S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohydr. Polym. 2014, 114, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cha, H.Y.; Seo, J.J.; Hong, J.T.; Han, K.; Oh, K.W. Anxiolytic-like effects of ginseng in the elevated plus-maze model: Comparison of red ginseng and sun ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Joh, E.H.; Kim, B.; Kim, D.H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of lps to toll-like receptor-4 on macrophages. Int. Immunopharmacol. 2012, 12, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M. Anti-inflammatory effects of ginsenosides Rg5, Rz1, and Rk1: Inhibition of tnf-alpha-induced nf-kappab, cox-2, and inos transcriptional expression. Phytother. Res. 2014, 28, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, A.K. Anti-breast cancer activity of fine black ginseng (Panax ginseng meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.W.; Bae, E.A.; Kim, D.H. Inhibitory effect of ginsenoside Rg5 and its metabolite ginsenoside Rh3 in an oxazolone-induced mouse chronic dermatitis model. Arch. Pharm. Res. 2006, 29, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jung, I.H.; Van Le, T.K.; Jeong, J.J.; Kim, N.J.; Kim, D.H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2013, 146, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, P.; Kim, T.; Ko, H.; Kim, H.K.; Kang, K.S.; Ham, J. Protective effects of processed ginseng and its active ginsenosides on cisplatin-induced nephrotoxicity: In vitro and in vivo studies. J. Agric. Food Chem. 2015, 63, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, J.Y.; Kim, S.O.; Yoo, Y.H.; Sung, S.H. Complete (1)h-nmr and (13)c-nmr spectral analysis of the pairs of 20(s) and 20(r) ginsenosides. J. Ginseng Res. 2014, 38, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Gu, J.; Feng, L.; Liu, J.; Zhang, M.; Jia, X.; Liu, M.; Yao, D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in stz-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014, 19, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Wang, Z.; Han, Y.; Tian, Y.H.; Zhang, G.S.; Sun, Y.S.; Wang, Y.P. Platycodin d isolated from the aerial parts of platycodon grandiflorum protects alcohol-induced liver injury in mice. Food Funct. 2015, 6, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, F.; Cao, X.; Zhai, Z.; GangHuang; Du, X.; Wang, Y.; Zhang, J.; Huang, Y.; Zhao, J.; et al. P2x7 receptor blockade protects against cisplatin-induced nephrotoxicity in mice by decreasing the activities of inflammasome components, oxidative stress and caspase-3. Toxicol. Appl. Pharmacol. 2014, 281, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, X.-M.; Han, Y.; Xu, Q.; Zhang, J.; Wang, Z.; Wang, Y.-P. Maltol, a maillard reaction product, exerts anti-tumor efficacy in h22 tumor-bearing mice via improving immune function and inducing apoptosis. RSC Adv. 2015, 5, 101850–101859. [Google Scholar] [CrossRef]
- Zhao, X.; Shu, G.; Chen, L.; Mi, X.; Mei, Z.; Deng, X. A flavonoid component from docynia delavayi (franch.) schneid represses transplanted h22 hepatoma growth and exhibits low toxic effect on tumor-bearing mice. Food Chem. Toxicol. 2012, 50, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Miao, L.; Zhang, H.; Yang, O.; Ge, H.; Li, Y.; Wang, L. Induction of interleukin 2 expression in the liver for the treatment of h22 hepatoma in mice. Dig. Liver Dis. 2013, 45, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Q.; He, Y.F.; Liu, Y.; Yang, S.B.; Wang, Z.; Zhang, J.; Zhao, L.C. Anti-tumor effect of steamed codonopsis lanceolata in h22 tumor-bearing mice and its possible mechanism. Nutrients 2015, 7, 8294–8307. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rojas, B.; Rodriguez-Rangel, D.S.; Granados-Castro, L.F.; Negrette-Guzman, M.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Molina-Jijon, E.; Reyes, J.L.; Zazueta, C.; Pedraza-Chaverri, J. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2015, 406, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.R.; Lee, S.H.; Jang, G.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 2014, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kim, H.; Joung, E.; Woo, K.; Jeong, J.; Yu, K.; Lee, J.; Jeong, H. Changes in ginsenosides and antioxidant activity of korean ginseng (Panax ginseng C.A. Meyer) with heating temperature and pressure. Food Sci. Biotechnol. 2010, 19, 941–949. [Google Scholar] [CrossRef]
- Lee, M.R.; Yun, B.S.; Sung, C.K. Comparative study of white and steamed black Panax ginseng, P. quinquefolium, and P. notoginseng on cholinesterase inhibitory and antioxidative activity. J. Ginseng Res. 2012, 36, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Park, J.S.; Jung, J.S.; Kim, D.H.; Kim, H.S. Anti-inflammatory effect of ginsenoside Rg5 in lipopolysaccharide-stimulated bv2 microglial cells. Int. J. Mol. Sci. 2013, 14, 9820–9833. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rojas, B.; Medina-Campos, O.N.; Hernandez-Pando, R.; Negrette-Guzman, M.; Huerta-Yepez, S.; Pedraza-Chaverri, J. C-phycocyanin prevents cisplatin-induced nephrotoxicity through inhibition of oxidative stress. Food Funct. 2014, 5, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.Y.; Zhang, J.; Yeo, S.J.; Myung, C.S.; Kim, H.M.; Kim, J.M.; Park, J.H.; Cho, J.; Kang, J.S. Memory enhancing and neuroprotective effects of selected ginsenosides. Arch. Pharm. Res. 2005, 28, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Cvijanovic, O.; Susnic, V.; Katalinic, N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology 2014, 324, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Jeong, J.Y.; Lim, B.J.; Chang, Y.K.; Lee, S.J.; Na, K.R.; Shin, Y.T.; Choi, D.E. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology 2009, 257, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kodama, A.; Watanabe, H.; Tanaka, R.; Kondo, M.; Chuang, V.T.; Wu, Q.; Endo, M.; Ishima, Y.; Fukagawa, M.; Otagiri, M.; et al. Albumin fusion renders thioredoxin an effective anti-oxidative and anti-inflammatory agent for preventing cisplatin-induced nephrotoxicity. Biochim. Biophys. Acta 2014, 1840, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Potocnjak, I.; Crncevic-Orlic, Z.; Skoda, M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem. Toxicol. 2014, 66, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kuncha, M.; Sindhura, G.J.; Sistla, R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 2013, 20, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Takahashi, N.; Shinohara, M.; Nakamura, K.; Mitazaki, S.; Abe, S.; Yoshida, M. Amelioration of cisplatin-induced mouse renal lesions by a cyclooxygenase (cox)-2 selective inhibitor. Eur. J. Pharmacol. 2013, 715, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Hussien, H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-alpha, interleukin 6, tumor suppressor p53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: Protective effect of ginseng. Food Chem. Toxicol. 2015, 78, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Tsuruya, K.; Ninomiya, T.; Tokumoto, M.; Hirakawa, M.; Masutani, K.; Taniguchi, M.; Fukuda, K.; Kanai, H.; Kishihara, K.; Hirakata, H.; et al. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003, 63, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Park, S.Y.; Kim, D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol. Pharm. Bull. 2000, 23, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.S.; Lee, H.J.; Bae, S.H.; Kim, S.Y.; Park, Y.; Suh, H.J.; Jeong, Y.H. The bioavailability of red ginseng extract fermented by phellinus linteus. J. Ginseng Res. 2013, 37, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.D.; He, T.; Du, T.W.; Fan, Y.G.; Chen, D.S.; Wang, Y. Ginsenoside Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol. Med. Rep. 2015, 11, 940–946. [Google Scholar] [CrossRef] [PubMed]








| Groups | Dosage (mg/kg) | Cisplatin Dosage (mg/kg) | Body Weight Change (g) | Kidney Index (mg/g) | BUN (mmol/L) | CRE (µmol/L) |
|---|---|---|---|---|---|---|
| Normal | — | +6.86 | 1.54 ± 0.14 | 7.53 ± 1.32 | 31.52 ± 2.14 | |
| Cisplatin | — | 25 | −2.80 * | 2.35 ± 0.15 * | 14.20 ± 2.11 ** | 201.34 ± 6.23 ** |
| Cisplatin + Rg5 | 10 | 25 | −1.09 | 1.91 ± 0.12 # | 12.80 ± 1.36 # | 97.96 ± 3.12 # |
| 20 | 25 | +0.64 # | 1.56 ± 0.14 ## | 11.70 ± 1.05 ## | 45.00 ± 2.15 ## |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yan, M.-H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.-S. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients 2016, 8, 566. https://doi.org/10.3390/nu8090566
Li W, Yan M-H, Liu Y, Liu Z, Wang Z, Chen C, Zhang J, Sun Y-S. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients. 2016; 8(9):566. https://doi.org/10.3390/nu8090566
Chicago/Turabian StyleLi, Wei, Meng-Han Yan, Ying Liu, Zhi Liu, Zi Wang, Chen Chen, Jing Zhang, and Yin-Shi Sun. 2016. "Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis" Nutrients 8, no. 9: 566. https://doi.org/10.3390/nu8090566
APA StyleLi, W., Yan, M. -H., Liu, Y., Liu, Z., Wang, Z., Chen, C., Zhang, J., & Sun, Y. -S. (2016). Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients, 8(9), 566. https://doi.org/10.3390/nu8090566