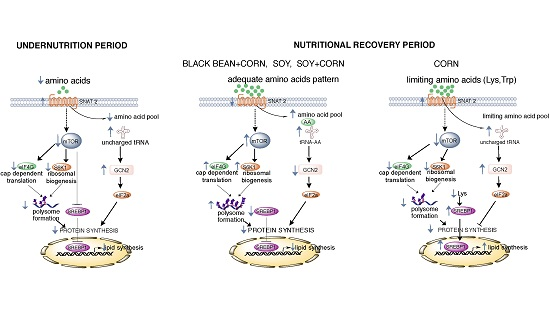
The mTORC1-Signaling Pathway and Hepatic Polyribosome Profile Are Enhanced after the Recovery of a Protein Restricted Diet by a Combination of Soy or Black Bean with Corn Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Protocol and Diets
2.1.1. Study 1: Effect of Different Types of Vegetable Proteins on Healthy Rats
2.1.2. Study 2: Effect of Different Types of Vegetable Proteins on Protein Restricted Rats
2.2. Biochemical Parameters
2.3. Determination of Plasma Amino Acids
2.4. Real-Time Quantitative PCR
2.5. Western Blot Analysis
2.6. Polyribosome Studies
2.7. Statistical Analysis
3. Results
3.1. Study 1
3.1.1. Weight Gain, Food Intake and Feed Conversion Ratio of Healthy Rats Fed Different Types of Proteins
3.1.2. Serum Biochemical Parameters and Homocysteine (Hcy) of Healthy Rats Fed Different Types of Proteins
3.1.3. Expression of the mTORC1-Signaling Pathway in the Livers of Healthy Rats Fed Different the Types of Proteins
3.1.4. Expression of the Amino Acid Transporter SNAT2 in the Liver of Healthy Rats Fed Different Types of Proteins
3.1.5. Liver Gene and Protein Expression of the Transcription Factor SREBP1c and Its Target Enzyme FASN of Healthy Rats Fed Different Types of Proteins
3.2. Study 2
3.2.1. Weight Gain, Food Intake and Feed Conversion Ratio of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.2. Serum Biochemical Variables of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.3. Plasma Amino Acids of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.4. Expression of the mTORC1-Signaling Pathway in the Livers of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.5. Expression of the Amino Acid Transporter SNAT2 in the Liver of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.6. Expression of GCN2 and eIF2α in the Liver of Rats Fed Different Types of Proteins after a Restricted Protein Period
3.2.7. Liver Gene and Protein Expression of the Transcription Factor SREBP1c and Its Target Enzyme FASN of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.8. Hepatic Polysome Profile of Rats Fed Different Types of Proteins after a Protein Restricted Period
3.2.9. Expression of the mTORC1-Signaling Pathway in the Muscles of Rats Fed Different Types of Proteins after a Protein Restricted Period
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldwin, C.; Parsons, T.; Logan, S. Dietary advice for illness-related malnutrition in adults. Cochrane Database Syst. Rev. 2001. [Google Scholar] [CrossRef]
- Grover, Z.; Ee, L.C. Protein energy malnutrition. Pediatr. Clin. N. Am. 2009, 56, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes. The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization; International Fund for Agricultural Development; World Food Programme. The State of Food in Security in the World 2015; FAO: Rome, Italy, 2015. [Google Scholar]
- Trabal, J.; Farran-Codina, A. Effects of dietary enrichment with conventional foods on energy and protein intake in older adults: A systematic review. Nutr. Rev. 2015, 73, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.K.; Smith, J.; Saljuqi, T.; Watling, R.M. Oral protein calorie supplementation for children with chronic disease. Cochrane Database Syst. Rev. 2015, 5, CD001914. [Google Scholar] [PubMed]
- Bressani, R.; Aguirrre, A.; Scrimshaw, N.S. All-vegetable protein mixtures for human feeding. II. The nutritive value of corn, sorghum, rice and buckwheat substituted for lime-treated corn in incap vegetable mixture eight. J. Nutr. 1959, 69, 351–355. [Google Scholar] [PubMed]
- Mensa-Wilmont, Y.; Phillips, R.D.; Hargrove, J.L. Protein quality evaluation of cowpea-based extrusion cooked cereal/legume weaning mixtures. Nutr. Res. 2001, 21, 849–857. [Google Scholar] [CrossRef]
- Scrimshaw, N.S.; Bressani, R.; Behar, M.; Viteri, F. Supplementation of cereal proteins with amino acids. I. Effect of amino acid supplementation of corn-masa at high levels of protein intake on the nitrogen retention of young children. J. Nutr. 1958, 66, 485–499. [Google Scholar] [PubMed]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; van Loon, L.J. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Nakayama, K.; Sanbongi, C.; Nagata, M.; Ikegami, S.; Itoh, H. Effects of whey, caseinate, or milk protein ingestion on muscle protein synthesis after exercise. Nutrients 2016, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, M.C.; Carnovale, C.E.; Favre, C.; Monti, J.A.; Scapini, C. Hepatic protein synthesis and serum aminoacid levels during liver regeneration in young and old malnourished rats. Mech. Ageing Dev. 1996, 91, 55–64. [Google Scholar] [CrossRef]
- Chotechuang, N.; Azzout-Marniche, D.; Bos, C.; Chaumontet, C.; Gausseres, N.; Steiler, T.; Gaudichon, C.; Tome, D. mTOR, Ampk, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1313–E1323. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, F.; Kimball, S.R.; Vary, T.C.; Jefferson, L.S. Effect of dietary protein on translation initiation in rat skeletal muscle and liver. Am. J. Physiol. 1998, 275, E814–E820. [Google Scholar] [PubMed]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Butteiger, D.N.; Cope, M.; Liu, P.; Mukherjea, R.; Volpi, E.; Rasmussen, B.B.; Krul, E.S. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin. Nutr. 2013, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Montalvo, I.; Sousa, V.; Sotelo, A. The protein efficiency ratios of 30:70 mixtures of animal:vegetable protein are similar or higher than those of the animal foods alone. J. Nutr. 1996, 126, 574–581. [Google Scholar] [PubMed]
- Le, L.T.; Sabate, J. Beyond meatless, the health effects of vegan diets: Findings from the adventist cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002, 269, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P.; Miyamoto, S. Nutrient-sensing mTORC1: Integration of metabolic and autophagic signals. J. Mol. Cell. Cardiol. 2016, 95, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Jiang, Y. Key factors in mtor regulation. Cell. Mol. Life Sci. 2010, 67, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, J.; Aledo, J.C.; Cwiklinski, E.; Hyde, R.; Taylor, P.M.; Hundal, H.S. SNAT2 transceptor signalling via mTOR: A role in cell growth and proliferation? Front. Biosci. 2011, 3, 1289–1299. [Google Scholar] [CrossRef]
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Rasmussen, B.B. Amino acid transporters in the regulation of human skeletal muscle protein metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Duvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. A new link in the chain from amino acids to mTORC1 activation. Mol. Cell 2011, 44, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Tokita, R.; Ohne, Y.; Hakuno, F.; Noguchi, T.; Minami, S.; Kato, H.; Takahashi, S. Dietary protein deprivation upregulates insulin signaling and inhibits gluconeogenesis in rat liver. J. Mol. Endocrinol. 2010, 45, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Averous, J.; Lambert-Langlais, S.; Mesclon, F.; Carraro, V.; Parry, L.; Jousse, C.; Bruhat, A.; Maurin, A.C.; Pierre, P.; Proud, C.G.; et al. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci. Rep. 2016, 6, 27698. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the Ain-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Torre-Villalvazo, I.; Tovar, A.R. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J. Nutr. Biochem. 2006, 17, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, F.; Brombo, G.; Zuliani, G. The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegener. Dis. Manag. 2016, 6, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Iadevaia, V.; Liu, R.; Proud, C.G. mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin. Cell Dev. Biol. 2014, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Vazquez-Pianzola, P. Functional diversity of the eukaryotic translation initiation factors belonging to eIF4 families. Mech. Dev. 2005, 122, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, V.; Zaklama, M.S.; Broquist, H.P. Dietary lysine and carnitine: Relation to growth and fatty livers in rats. J. Nutr. 1976, 106, 111–117. [Google Scholar] [PubMed]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. Srebp transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Palii, S.S.; Chen, H.; Kilberg, M.S. Transcriptional control of the human sodium-coupled neutral amino acid transporter system a gene by amino acid availability is mediated by an intronic element. J. Biol. Chem. 2004, 279, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yamamoto, A.; Fujita, T. Functional expression and adaptive regulation of Na+ -dependent neutral amino acid transporter SNAT2/ATA2 in normal human astrocytes under amino acid starved condition. Neurosci. Lett. 2005, 378, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [PubMed]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, C.; Torres, N.; Isoard-Acosta, F.; Gomez-Perez, F.J.; Hernandez-Pando, R.; Tovar, A.R. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J. Nutr. 2004, 134, 522–529. [Google Scholar] [PubMed]
- Blum, A.; Hijazi, I.; Eizenberg, M.M.; Blum, N. Homocysteine (hcy) follow-up study. Clin. Investg. Med. 2007, 30, 21–25. [Google Scholar]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant protein and animal proteins: Do they differentially affect cardiovascular disease risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Bressani, R. Incap studies of vegetable proteins for human consumption. Food Nutr. Bull. 2010, 31, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J.; Waterlow, J.C.; Swick, R.W. Measurement of protein turnover in rat liver. Analysis of the complex curve for decay of label in a mixture of proteins. Biochem. J. 1976, 156, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J.; Garlick, P.J.; Stewart, R.J.; Nnanyelugo, D.O.; Waterlow, J.C. Skeletal-muscle growth and protein turnover. Biochem. J. 1975, 150, 235–243. [Google Scholar] [CrossRef] [PubMed]










| Ingredients | C | LP | S | B | P | Sp | Se | Cr | BCr | SCr |
|---|---|---|---|---|---|---|---|---|---|---|
| Casein 1, g | 219.8 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Soy protein isolate 2, g | 0 | 0 | 220 | 0 | 0 | 0 | 0 | 0 | 0 | 110.2 |
| Black bean protein concentrate 3, g | 0 | 0 | 0 | 287.5 | 0 | 0 | 0 | 0 | 144.3 | 0 |
| Pea protein isolate 4, g | 0 | 0 | 0 | 0 | 275.3 | 0 | 0 | 0 | 0 | 0 |
| Spirulina 5, g | 0 | 0 | 0 | 0 | 0 | 410.3 | 0 | 0 | 0 | 0 |
| Sesame protein isolate 6, g | 0 | 0 | 0 | 0 | 0 | 0 | 232 | 0 | 0 | 0 |
| Corn protein isolate 7, g | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 336.2 | 167.4 | 167.9 |
| Corn starch, g | 390.4 | 520.4 | 389.9 | 371.7 | 373 | 331.8 | 386.6 | 352.6 | 362.2 | 371.3 |
| Dextrinized cornstarch, g | 126 | 172.7 | 127 | 110.2 | 111.4 | 69.7 | 126.2 | 89 | 99.6 | 108 |
| Sucrose, g | 94.2 | 130.8 | 95.3 | 78.7 | 79.8 | 38.1 | 94.8 | 57.2 | 68 | 76.3 |
| Soybean oil, g | 69.5 | 70 | 68.2 | 55.8 | 61.9 | 39.9 | 62.6 | 66.1 | 61 | 67.2 |
| α-Cellulose, g | 49.8 | 50 | 49.5 | 46.4 | 48.9 | 47.7 | 48.2 | 48.8 | 47.6 | 49.2 |
| AIN-93-MX, g | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| AIN-93-VX, g | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| l-Cystine, g | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Choline bitartrate, g | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| TBHQ, g | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Total amount, g | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Total protein content, % kcal | 19.3 | 0.5 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 |
| Total carbohydrate content, % kcal | 65.5 | 84.3 | 65.5 | 65.5 | 65.5 | 65.5 | 65.5 | 65.5 | 65.5 | 65.5 |
| Total fat content, % kcal | 15.2 | 15.2 | 15.2 | 15.2 | 15.2 | 15.2 | 15.2 | 15.2 | 15.2 | 15.2 |
| Energy density, kcal/g | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 |
| C | S | B | BCr | P | Sp | Se | Cr | P 1 | P 2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial body weight, g | 200.2 ± 1.3 b | 193.5 ± 2.5 bc | 195.5 ± 1.5 b | 197.1 ± 2.1 b | 186.7 ± 1.6 c | 199.3 ± 1.9 b | 199.8 ± 2.1 b | 212.5 ± 1.9 a | 0.001 | 0.001 |
| Final body weight, g | 255.4 ± 1.9 a | 239.8 ± 3.3 bc | 236.7 ± 2.3 bd | 248.9 ± 2.2 ac | 245.0 ± 3.0 ac | 228.4 ± 2.7 d | 225.9 ± 2.0 d | 229.6 ± 2.4 d | 0.001 | 0.001 |
| Weight gain, day 4–21, g | 55.2 ± 1.9 ab | 46.3 ± 2.3 b | 41.2 ± 2.2 c | 51.8 ± 2.3 bc | 58.3 ± 2.6 a | 29.2 ± 2.9 d | 26.1 ± 2.0 de | 17.1 ± 1.6 de | ||
| Food intake, g/day | 19.0 ± 0.9 a | 16.8 ± 0.8 ab | 17.4 ± 0.4 a | 15.5 ± 0.5 b | 15.4 ± 1.0 b | 16.1 ± 0.7 a | 14.2 ± 0.4 b | 15.8 ± 0.5 b | ||
| Feed conversion ratio (FCR) | 2.9 ± 0.1 bc | 2.7 ± 0.2 c | 2.4 ± 0.1 cd | 3.4 ± 0.2 ab | 3.9 ± 0.2 a | 1.8 ± 0.2 d | 1.8± 0.2 d | 1.1 ± 0.1 e | ||
| Serum | ||||||||||
| Glucose, mmol/L | 7.2 ± 0.3 ab | 6.8 ± 0.2 b | 6.2 ± 0.2 b | 7.0 ± 0.1 ab | 7.9 ± 0.3 a | 6.1 ± 0.4 b | 7.4 ± 0.2 a | 7.5 ± 0.2 a | ||
| Triglycerides, mmol/L | 0.28 ± 0.1 b | 0.51 ± 0.1 ab | 0.5 ± 0.1 ab | 0.61 ± 0.1 ab | 0.58 ± 0.1 ab | 0.71 ± 0.1 a | 0.40 ± 0.1 ab | 0.63 ± 0.1 ab | ||
| Cholesterol, mmol/L | 1.64 ± 0.50 b | 1.60 ± 0.06 b | 1.54 ± 0.05 b | 1.77 ± 0.12 b | 1.58 ± 0.07 b | 1.79 ± 0.07 a | 2.11 ± 0.10 a | 2.14 ± 0.06 a | ||
| Insulin, pmol/L | 269.9 ± 5.2 a | 158.7 ± 3.7 cd | 55.8 ± 5.6 e | 160.5 ± 5.8 c | 167.0 ± 5.7 c | 168.4 ± 6.3 c | 204.7 ± 7.3 b | 134.0 ± 3.5 d | ||
| Glucagon, ng/L | 75.3 ± 2.3 bc | 70.1 ± 1.1 bc | 103.3 ± 2.4 a | 70.3 ± 3.5 bc | 74.6 ± 3.3 bc | 65.2 ± 5.3 c | 44.9 ± 2.9 d | 86.4 ± 5.8 b | ||
| Insulin/Glucagon | 3.8 ± 0.3 a | 2.3 ± 0.1 bc | 0.6 ± 0.1 d | 2.2 ± 0.1 bc | 2.3 ± 0.1 bc | 2.7 ± 0.3 b | 4.6 ± 0.3 a | 1.5 ± 0.1 cd |
| LP | C | S | B | BCr | SCr | Cr | P 1 | P 2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial body weight, g | 147.3 ± 2.0 a | 145.3 ± 1.6 abc | 144.6 ± 1.9 abc | 151.7 ± 1.6 a | 140.9 ± 1.5 bc | 137.5 ± 1.8 c | 143.2 ± 2.1 bc | 0.048 | 0.001 | |
| Body weight, day 7, g | 114.7 ± 1.5 d | 157.7 ± 1.9 a | 138.6 ± 2.6 bc | 140.3 ± 2.6 bc | 154.7 ± 4.5 a | 149.2 ± 2.5 ab | 133.4 ± 2.1 c | 0.048 | 0.001 | |
| Food intake, g/day | 8.39 ± 0.6 b | 18.22 ± 1.3 a | 15.82 ± 0.9 a | 14.44 ± 0.9 a | 17.61 ± 0.9 a | 17.96 ± 1.5 a | 10.64 ± 0.6 b | |||
| Feed conversion ratio, day 7 | −3.7 ± 0.4 d | 0.8 ± 0.2 a | 0.9 ± 0.2 a | −0.3 ± 0.1 bc | −0.1 ± 0.1 b | −1.1 ± 0.2 c | −1.0 ± 0.1 d | |||
| Biochemical parameters | Day | |||||||||
| Glucose, mmol/L | 7 | 8.5 ± 0.5 ab | 9.3 ± 0.2 a | 8.6 ± 0.5 ab | 7.8 ± 0.2 bc | 7.4 ± 0.2 bc | 7.0 ± 0.3 c | 7.7 ± 0.2 bc | ||
| Triglycerides, mmol/L | 7 | 0.77 ± 0.1 b | 0.58 ± 0.2 b | 1.28 ± 0.1 a | 0.72 ± 0.1 b | 1.15 ± 0.1 a | 0.92 ± 0.1 b | 0.85 ± 0.1 b | ||
| Cholesterol, mmol/L | 7 | 1.66 ± 0.1 b | 2.73 ± 0.1 a | 2.71 ± 0.1 a | 1.96 ± 0.1 b | 2.38 ± 0.2 a | 2.39 ± 0.2 a | 2.52 ± 0.1 a | ||
| Insulin, pmol/L | 7 | 83.5 ± 6.8 d | 445.0 ± 15.3 a | 218.5 ± 16.5 b | 148.1 ± 10.9 c | 230.3 ± 12.9 b | 249.5 ± 16.9 b | 239.0 ± 13.8 b | ||
| Glucagon, ng/L | 7 | 74.3 ± 3.7 c | 88.9 ± 6.3 bc | 99.0 ± 4.7 ab | 110.2 ± 2.2 a | 76.3 ± 3.2 bc | 114.1 ± 3.8 a | 105.2 ± 3.8 ab | ||
| Insulin/Glucagon | 7 | 1.1 ± 0.1 c | 5.1 ± 0.4 a | 2.3 ± 0.3 bc | 1.4 ± 0.1 c | 3.0 ± 0.2 b | 2.2 ± 0.2 bc | 2.3 ± 0.1 bc | ||
| Albumin g/dL | Δ7-1 | −0.25 ± 0.07 d | 1.60 ± 0.11 a | 1.32 ± 0.15 ab | 1.05 ± 0.24 ab | 0.73 ± 0.12 b | 0.66 ± 0.23 b | 0.30 ± 0.16 c | ||
| AMINO ACIDS (µmol/mL) | LP | C | S | B | BCr | SCr | Cr |
|---|---|---|---|---|---|---|---|
| Asp | 0.045 ± 0.001 c | 0.078 ± 0.003 a | 0.061 ± 0.002 b | 0.057 ± 0.003 bc | 0.055 ± 0.004 bc | 0.059 ± 0.002 bc | 0.056 ± 0.03 bc |
| Glu | 0.24 ±0.008 b | 0.26 ±0.006 a | 0.24 ± 0.005 b | 0.23 ± 0.001 b | 0.17± 0.003 c | 0.23 ± 0.006 b | 0.18 ± 0.004 c |
| Ser | 0.56 ± 0.01 c | 0.92 ± 0.03 ab | 1.08 ± 0.06 a | 0.88 ± 0.02 b | 0.83 ± 0.02 b | 0.97 ± 0.03 ab | 0.91 ± 0.05 ab |
| His | 0.055 ± 0.001 b | 0.100 ± 0.006 a | 0.095 ± 0.004 a | 0.079 ± 0.004 ab | 0.060 ± 0.005 b | 0.102 ± 0.008 a | 0.063 ± 0.006 b |
| Gly | 0.76 ± 0.01 c | 0.74 ± 0.02 c | 0.94 ± 0.02 b | 1.08 ± 0.02 a | 0.66 ± 0.02 dc | 0.65 ± 0.02 dc | 0.56 ± 0.02 d |
| Thr | 0.19 ± 0.03 b | 0.27 ± 0.05 ab | 0.37 ± 0.06 ab | 0.37 ± 0.02 ab | 0.31 ± 0.07 ab | 0.41 ± 0.05 a | 0.46 ± 0.02 a |
| Arg | 0.02 ± 0.002 c | 0.19 ± 0.001 a | 0.12 ± 0.016 b | 0.15 ± 0.011 ab | 0.14 ± 0.012 b | 0.21 ± 0.004 a | 0.16 ± 0.015 ab |
| Ala | 1.53 ± 0.03 cd | 1.68 ± 0.05 bc | 2.13 ± 0.06 a | 1.78 ± 0.04 b | 1.34 ± 0.05 d | 1.50 ± 0.07 cd | 1.39 ± 0.02 d |
| Val | 0.070 ± 0.006 d | 0.324 ± 0.016 a | 0.271 ± 0.009 ab | 0.242 ± 0.012 bc | 0.205 ± 0.008 c | 0.237 ± 0.013 bc | 0.215 ± 0.009 c |
| Met | 0.11 ± 0.009 ab | 0.15 ± 0.010 a | 0.11 ± 0.007 ab | 0.10 ± 0.007 b | 0.10 ± 0.005 b | 0.12 ± 0.009 ab | 0.12 ± 0.010 ab |
| Cys | 0.607 ± 0.004 c | 0.695 ± 0.013 b | 0.891 ± 0.027 a | 0.768 ± 0.016 b | 0.528 ± 0.008 de | 0.575 ± 0.012 d | 0.471 ± 0.022 e |
| Phe | 0.043 ± 0.004 b | 0.111 ± 0.009 a | 0.111 ± 0.005 a | 0.109 ± 0.008 a | 0.108 ± 0.003 a | 0.113 ± 0.006 a | 0.119 ± 0.006 a |
| Tyr | 0.040 ± 0.002 d | 0.185 ± 0.005 ab | 0.170 ± 0.008 ab | 0.140 ± 0.008 c | 0.145 ± 0.005 bc | 0.190 ± 0.015 a | 0.191 ± 0.003 a |
| Ile | 0.046 ± 0.002 d | 0.118 ± 0.004 a | 0.123 ± 0.04 a | 0.096 ± 0.002 b | 0.072 ± 0.03 c | 0.083 ± 0.05 bc | 0.066 ± 0.004 c |
| Leu | 0.060 ± 0.005 d | 0.201 ± 0.006 b | 0.179 ± 0.008 bc | 0.156 ± 0.003 c | 0.201 ± 0.011 b | 0.230 ± 0.011 a | 0.259 ± 0.012 a |
| Lys | 0.30 ± 0.006 cd | 0.49 ± 0.012 ab | 0.51 ± 0.022 a | 0.42 ± 0.012 b | 0.28 ± 0.016 d | 0.35 ± 0.012 bc | 0.17 ± 0.014 e |
| Pro | 0.31 ± 0.01 c | 0.68 ± 0.02 a | 0.48 ± 0.001 b | 0.34 ± 0.02 c | 0.45 ± 0.01 b | 0.39 ± 0.02 c | 0.51 ± 0.001 b |
| Trp | 0.036 ± 0.003 b | 0.093 ± 0.008 a | 0.078 ± 0.004 a | 0.031 ± 0.002 b | 0.024 ± 0.003 b | 0.037 ± 0.005 b | 0.018 ± 0.003 c |
| Asn | 0.032 ± 0.001 c | 0.065 ± 0.03 b | 0.116 ± 0.007 a | 0.70 ± 0.004 b | 0.75 ± 0.005 b | 0.108 ± 0.03 a | 0.081 ± 0.001 b |
| Gln | 0.81 ± 0.01 c | 0.89 ± 0.001 a | 0.88 ± 0.001 a | 0.84 ± 0.001 bc | 0.72 ± 0.001 d | 0.87 ± 0.01 ab | 0.71 ± 0.01 d |
| ΣBCAA | 0.526 ± 0.004 d | 1.929 ± 0.03 a | 1.722 ± 0.022 b | 1.481 ± 0.022 c | 1.432 ± 0.022 c | 1.653 ± 0.026 b | 1.620 ± 0.030 b |
| ΣIAA | 1.909 ± 0.01 e | 4.666 ± 0.02 a | 3.993 ± 0.02 c | 3.994 ± 0.02 c | 3.684 ± 0.02 d | 4.692 ± 0.02 a | 4.462 ± 0.03 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Mota, C.C.; Rodriguez-Gaytan, C.; Adjibade, P.; Mazroui, R.; Gálvez, A.; Granados, O.; Tovar, A.R.; Torres, N. The mTORC1-Signaling Pathway and Hepatic Polyribosome Profile Are Enhanced after the Recovery of a Protein Restricted Diet by a Combination of Soy or Black Bean with Corn Protein. Nutrients 2016, 8, 573. https://doi.org/10.3390/nu8090573
Márquez-Mota CC, Rodriguez-Gaytan C, Adjibade P, Mazroui R, Gálvez A, Granados O, Tovar AR, Torres N. The mTORC1-Signaling Pathway and Hepatic Polyribosome Profile Are Enhanced after the Recovery of a Protein Restricted Diet by a Combination of Soy or Black Bean with Corn Protein. Nutrients. 2016; 8(9):573. https://doi.org/10.3390/nu8090573
Chicago/Turabian StyleMárquez-Mota, Claudia C., Cinthya Rodriguez-Gaytan, Pauline Adjibade, Rachid Mazroui, Amanda Gálvez, Omar Granados, Armando R. Tovar, and Nimbe Torres. 2016. "The mTORC1-Signaling Pathway and Hepatic Polyribosome Profile Are Enhanced after the Recovery of a Protein Restricted Diet by a Combination of Soy or Black Bean with Corn Protein" Nutrients 8, no. 9: 573. https://doi.org/10.3390/nu8090573
APA StyleMárquez-Mota, C. C., Rodriguez-Gaytan, C., Adjibade, P., Mazroui, R., Gálvez, A., Granados, O., Tovar, A. R., & Torres, N. (2016). The mTORC1-Signaling Pathway and Hepatic Polyribosome Profile Are Enhanced after the Recovery of a Protein Restricted Diet by a Combination of Soy or Black Bean with Corn Protein. Nutrients, 8(9), 573. https://doi.org/10.3390/nu8090573