Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders
Abstract
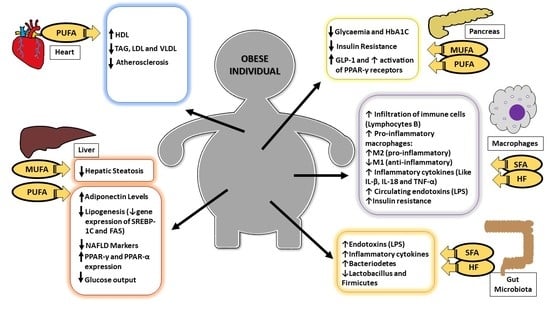
:1. Introduction
2. Insulin Resistance and Associated Comorbidities
3. Dyslipidemias
4. Inflammatory Process and Intestinal Microbiota
5. Fatty Acids and Non-Alcoholic Fatty Liver Disease
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lyons, C.; Kennedy, E.; Roche, H. Metabolic inflammation-differential modulation by dietary constituents. Nutrients 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.C.W.; Dhillon, B.; Yan, H.; Szmitko, P.E.; Verma, S. Adipokines: Molecular links between obesity and atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2031–H2041. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Bing, C.; Wood, I.S. Adipose tissue and adipokines—Energy regulation from the human perspective. J. Nutr. 2006, 136, 1935S–1939S. [Google Scholar] [PubMed]
- Xiao, L.; Yang, X.; Lin, Y.; Li, S.; Jiang, J.; Qian, S.; Tang, Q.; He, R.; Li, X. Large adipocytes function as antigen-presenting cells to activate cd4+ t cells via upregulating mhcii in obesity. Int. J. Obes. 2016, 40, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Pillon, N.J.; Sivaloganathan, D.M.; Costford, S.R.; Liu, Z.; Théret, M.; Chazaud, B.; Klip, A. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via amp-activated protein kinase (AMPK). J. Biol. Chem. 2015, 290, 16979–16988. [Google Scholar] [CrossRef] [PubMed]
- Kien, C. Dietary interventions for metabolic syndrome: Role of modifying dietary fats. Curr. Diabetes Rep. 2009, 9, 43–50. [Google Scholar] [CrossRef]
- Giacca, A.; Xiao, C.; Oprescu, A.I.; Carpentier, A.C.; Lewis, G.F. Lipid-induced pancreatic β-cell dysfunction: Focus on in vivo studies. Am. J. Physiol. Endocrinol. Metab. 2011. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A.; Dhillon, S. Omega-3 carboxylic acids (epanova): A review of its use in patients with severe hypertriglyceridemia. Am. J. Cardiovasc. Drugs 2014. [Google Scholar] [CrossRef] [PubMed]
- Crandell, J.R.; Tartaglia, C.; Tartaglia, J. Lipid effects of switching from prescription epa+dha (omega-3-acid ethyl esters) to prescription epa-only (icosapent ethyl) in dyslipidemic patients. Postgrad. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.M.M.; Watts, G.F.; Ng, T.W.K.; Hugh, P.; Barrett, R. Effect of dietary fatty acids on human lipoprotein metabolism: A comprehensive update. Nutrients 2015, 7, 4416–4425. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 2002, 27, 467–473. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar]
- Arunima, S.; Rajamohan, T. Influence of virgin coconut oil-enriched diet on the transcriptional regulation of fatty acid synthesis and oxidation in rats—A comparative study. Br. J. Nutr. 2014, 111, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.E.; Nair, K.S.; Jensen, M.D. Insulin regulation of regional free fatty acid metabolism. Diabetes 1999, 48, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Riserus, U. Fatty acids and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 2008. [Google Scholar] [CrossRef] [PubMed]
- Koska, J.; Ozias, M.K.; Deer, J.; Kurtz, J.; Salbe, A.D.; Harman, S.M.; Reaven, P.D. A human model of dietary saturated fatty acid induced insulin resistance. Metab. Clin. Exp. 2016, 65, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qian, S.Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014. [Google Scholar] [CrossRef]
- Bermudez, B.; Ortega-Gomez, A.; Varela, L.M.; Villar, J.; Abia, R.; Muriana, F.J.G.; Lopez, S.; Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.; et al. Clustering effects on postprandial insulin secretion and sensitivity in response to meals with different fatty acid compositions. Food Funct. 2014, 5, 1374. [Google Scholar] [CrossRef] [PubMed]
- Shadman, Z.; Khoshniat, M.; Poorsoltan, N.; Akhoundan, M.; Omidvar, M.; Larijani, B.; Hoseini, S. Association of high carbohydrate versus high fat diet with glycated hemoglobin in high calorie consuming type 2 diabetics. J. Diabetes Metab. Disord. 2013, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Crochemore, I.C.C.; Souza, A.F.P.; de Souza, A.C.F.; Rosado, E.L. Ω-3 polyunsaturated fatty acid supplementation does not influence body composition, insulin resistance, and lipemia in women with type 2 diabetes and obesity. Nutr. Clin. Pract. 2012, 27, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Müllner, E.; Plasser, E.; Brath, H.; Waldschütz, W.; Forster, E.; Kundi, M.; Wagner, K.H. Impact of polyunsaturated vegetable oils on adiponectin levels, glycaemia and blood lipids in individuals with type 2 diabetes: A randomised, double-blind intervention study. J. Hum. Nutr. Diet. 2014, 27, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; Kouba, E.O.; Chilton, F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.M.; Hollanda-Miranda, W.R.; Beraldo, R.A.; Castro, A.V.B.; Geloneze, B.; Foss, M.C.; Foss-Freitas, M.C. Supplementation of α-linolenic acid improves serum adiponectin levels and insulin sensitivity in patients with type 2 diabetes. Nutrition 2015, 31, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Bozzeto, L.; Prinster, A.; Costagliola, L.; Mangione, A.; Vitelli, A. Liver fat is reduced by an isoenergetic mufa diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 2012, 35, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Garibay-Nieto, N.; Queipo-Garcia, G.; Alvarez, F.; Bustos, M.; Villanueva, E.; Ramirez, F.; Leon, M.; Laresgoiti-Servitje, E.; Duggirala, R.; Macias, T.; et al. Effects of conjugated linoleic acid and metformin on insulin sensitivity in obese children: Randomized clinical trial. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Lee, J.J.; Kim, Y.; Kim, I.S.; Park, J.S.; Myung, C.S. Amelioration of insulin resistance by scopoletin in high-glucose-induced, insulin-resistant hepg2 cells. Horm. Metab. Res. 2010, 42, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Vaag, A.A.; Holst, J.J.; Volund, A.; Becknielsen, H. Gut incretin hormones in identical-twins discordant for non- insulin-dependent diabetes-mellitus (niddm)—Evidence for decreased glucagon-like peptide-1 secretion during oral glucose- ingestion in niddm twins. Eur. J. Endocrinol. 1996, 135, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-R.; Sun, C.-H.; Liu, J.-R.; Zhao, D. Dietary conjugated linoleic acid increases ppar gamma gene expression in adipose tissue of obese rat, and improves insulin resistance. Growth Horm. IGF Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, Ó.; Díaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Griffiths, H.R.; Bailey, C.J. Oleate protects against palmitate-induced insulin resistance in l6 myotubes. Br. J. Nutr. 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Tang, Y.-S.; Song, Y.-L.; Li, A.; Zhou, H.; Li, Y. Saturated fatty acid induces insulin resistance partially through nucleotide-binding oligomerization domain 1 signaling pathway in adipocytes. Chin. Med. Sci. J. 2013, 28, 211–217. [Google Scholar] [CrossRef]
- Malinska, H.; Huttl, M.; Oliyarnyk, O.; Bratova, M.; Kazdova, L. Conjugated linoleic acid reduces visceral and ectopic lipid accumulation and insulin resistance in chronic severe hypertriacylglycerolemia. Nutrition 2015. [Google Scholar] [CrossRef] [PubMed]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; McAllan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose nlrp3 inflammasome-mediated il-1β secretion and insulin resistance despite obesity. Diabetes 2015. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.S.; Lagreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 2001. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.S.; Brubaker, P.L. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 1995. [Google Scholar] [CrossRef] [PubMed]
- Lucero, D.; Olano, C.; Bursztyn, M.; Morales, C.; Stranges, A.; Friedman, S.; Macri, E.V.; Schreier, L.; Zago, V. Supplementation with n-3, n-6, n-9 fatty acids in an insulin-resistance animal model: Does it improve vldl quality? Food Funct. 2017, 8, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Sambra Vásquez, V.; Rojas Moncada, P.; Basfi-Fer, K.; Valencia, A.; Codoceo, J.; Inostroza, J.; Carrasco, F.; Ruz Ortiz, M. Impact of dietary fatty acids on lipid profile, insulin sensitivity and functionality of pancreatic β cells in type 2 diabetic subjects. Nutrición Hospitalaria 2015, 32, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.H.; Kraegen, E.W.; Chisholm, D.J.; Ford, G.L.; Bruce, D.G.; Pascoe, W.S.; Chisholm, D.J.; Ford, G.L.; Bruce, D.G.; Pascoe, W.S. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987, 237, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Neves Ribeiro, D.; De Cássia, R.; Alfenas, G.; Bressan, J.; Brunoro Costa, N.M. The effect of oilseed consumption on appetite and on the risk of developing type 2 diabetes mellitus. Nutr. Hosp. 2013, 28, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C.; Nälsén, C.; Berglund, L.; Louheranta, A.; Rasmussen, B.M.; et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The kanwu study. Diabetologia 2001. [Google Scholar] [CrossRef]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Moran, M.; Guerrero-Romero, F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects. Diabetes Care 2003, 26, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Kaline, K.; Bornstein, S.R.; Bergmann, A.; Hauner, H.; Schwarz, P.E.H. The importance and effect of dietary fiber in diabetes prevention with particular consideration of whole grain products. Horm. Metab. Res. 2007, 39, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B. Fiber and magnesium intake and incidence of type 2 diabetes. Arch. Intern. Med. 2007. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Elliott, E.J.; Baur, L. Low glycaemic index, or low glycaemic load, diets for overweight and obesity. Cochrane Libr. 2005. [Google Scholar] [CrossRef]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [PubMed]
- Okuyama, H.; Langsjoen, P.H.; Ohara, N.; Hashimoto, Y.; Hamazaki, T.; Yoshida, S.; Kobayashi, T.; Langsjoen, A.M. Medicines and vegetable oils as hidden causes of cardiovascular disease and diabetes. Pharmacology 2016, 98, 134–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Samarghandi, A.; Zhang, N.; Yao, Z.; Xiong, M.; Teng, B.B. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein b and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjaerg-Hansen, A. Loss-of-function mutations in apoc3 and risk of ischemic vascular disease. N. Engl. J. Med. 2014, 371, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Kathiresan, S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ. Res. 2016, 118, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Hurtubise, J.; McLellan, K.; Durr, K.; Onasanya, O.; Nwabuko, D.; Ndisang, J.F. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr. Atheroscler. Rep. 2016, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Sanin, V.; Pfetsch, V.; Koenig, W. Dyslipidemias and cardiovascular prevention: Tailoring treatment according to lipid phenotype. Curr. Cardiol. Rep. 2017, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Meigs, J.B.; Manson, J.E.; Rifai, N.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 2005, 135, 562–566. [Google Scholar] [PubMed]
- Benes, L.B.; Bassi, N.S.; Davidson, M.H. Omega-3 carboxylic acids monotherapy and combination with statins in the management of dyslipidemia. Vasc. Health Risk Manag. 2016. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arora, R.R.; Singh, M.; Khosla, S. Eicosapentaenoic acid versus docosahexaenoic acid as options for vascular risk prevention. Am. J. Therap. 2016, 23, e905–e910. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish oil—How does it reduce plasma triglycerides? Biochimica et Biophysica Acta 2012, 1821, 843–851. [Google Scholar] [CrossRef] [PubMed]
- De Mattos, A.M.; da Costa, J.A.C.; Jordão Júnior, A.A.; Chiarello, P.G. Omega-3 fatty acid supplementation is associated with oxidative stress and dyslipidemia, but does not contribute to better lipid and oxidative status on hemodialysis patients. J. Renal Nutr. 2017, 27, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Tsubata, H.; Hashimoto, N.; Takabe, M.; Miyata, T.; Aoki, K.; Yamashita, S.; Oishi, S.; Osue, T.; Yokoi, K.; et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc. Diabetol. 2016. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhu, Y.; Liu, X.; Xia, H.; Yang, X.; Sun, G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: A randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Jahreis, G.; Bothor, K.; Drechsel, C.; Kiehntopf, M.; Blüher, M.; Dawczynski, C. Benefits of foods supplemented with vegetable oils rich in α-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomized, controlled trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Motevalli, M.; Westphal, D.; Kwiterovich, P.O. Incorporation of oleic acid and eicosapentaenoic acid into glycerolipids of cultured normal human fibroblasts. Lipids 1993, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Wang, Y.; Parhar, R.S.; Collison, K.S.; Conca, W.; Al-Mohanna, F.; Gaugler, R. A c. Elegans model to study human metabolic regulation. Nutr. Metab. (Lond.) 2013, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V.; Novgorodtseva, T.P.; Denisenko, Y.K.; Gonzalez, D.E.; Mustad, V.A.; Kris-Etherton, P.M.; Rise, P.; Eligini, S.; Ghezzi, S.; Colli, S.; et al. Effect of the prolonged high-fat diet on the fatty acid metabolism in rat blood and liver. Lipids Health Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Barrows, B.R.; Parks, E.J. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J. Clin. Endocrinol. Metab. 2006, 91, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Parlevliet, E.T.; Wang, Y.; Geerling, J.J.; Schröder-Van der Elst, J.P.; Picha, K.; O’Neil, K.; Stojanovic-Susulic, V.; Ort, T.; Havekes, L.M.; Romijn, J.A.; et al. Glp-1 receptor activation inhibits vldl production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed apoe*3-leiden mice. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.S.; Candido, C.J.; Jaques, J.A.S.; Nunes, Â.A.; Caires, A.R.L.; Michels, F.S.; Almeida, J.A.; Filiú, W.F.O.; Hiane, P.A.; Nascimento, V.A.; et al. Oxidative stability of sesame and flaxseed oils and their effects on morphometric and biochemical parameters in an animal model. J. Sci. Food Agric. 2017, 97, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low n-6/n-3 pufa ratio improves lipid metabolism, inflammation, oxidative stress and endothelial function in rats using plant oils as n-3 fatty acid source. Lipids 2016, 51, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Duan, Y.; Li, Y.; Tang, Y.; Geng, M.; Oladele, O.A.; Kim, S.W.; Yin, Y. Effects of dietary n-6:N-3 pufa ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Macri, E.V.; Lifshitz, F.; Alsina, E.; Juiz, N.; Zago, V.; Lezón, C.; Rodriguez, P.N.; Schreier, L.; Boyer, P.M.; Friedman, S.M. Monounsaturated fatty acids-rich diets in hypercholesterolemic-growing rats. International J. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alsina, E.; Macri, E.V.; Lifshitz, F.; Bozzini, C.; Rodriguez, P.N.; Boyer, P.M.; Friedman, S.M. Efficacy of phytosterols and fish-oil supplemented high-oleic-sunflower oil rich diets in hypercholesterolemic growing rats. Int. J. Food Sci. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Giudetti, A.M. Dietary long-chain unsaturated fatty acids acutely and differently reduce the activities of lipogenic enzymes and of citrate carrier in rat liver. J. Physiol. Biochem. 2016, 72, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.W.; Davis, H.R.; Zhu, L.-J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-pick c1 like 1 protein is critical for intestinal cholesterol absorption. Science (N. Y.) 2004. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tanaka, K.; Sugano, M.; Vahouny, G.V.; Gallo, L.L. Inhibition of cholesterol absorption in rats by plant sterols. J. Lipid Res. 1988, 29, 1573–1582. [Google Scholar] [PubMed]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Eissing, L.; Scherer, T.; Todter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to chrebp-beta and metabolic health. Nat. Commun. 2013, 4, 1528. [Google Scholar] [CrossRef] [PubMed]
- Borkman, M.; Storlien, L.H.; Pan, D.A.; Jenkins, A.B.; Chisholm, D.J.; Campbell, L.V. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N. Engl. J. Med. 1993. [Google Scholar] [CrossRef] [PubMed]
- Rustan, A.C. Fatty acids: Structures and properties. Encycl. Life Sci. 2009. [Google Scholar] [CrossRef]
- Caër, C.; Rouault, C.; Roy, T.L.; Poitou, C.; Aron, J.; Adriana, T.; Bic, J.-C.; Clément, K. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. 2017. [Google Scholar] [CrossRef]
- Moya-Pérez, A.; Neef, A.; Sanz, Y. Bifidobacterium pseudocatenulatum cect 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Rainone, V.; Schneider, L.; Saulle, I.; Ricci, C.; Biasin, M.; Al-Daghri, N.M.; Giani, E.; Zuccotti, G.V.; Clerici, M.; Trabattoni, D. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int. J. Obes. 2016, 40, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Lyte, J.M.; Gabler, N.K.; Hollis, J.H. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis. 2016, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Bunn, J.Y.; Fukagawa, N.K.; Anathy, V.; Matthews, D.E.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Pratley, R.E.; Poynter, M.E. Lipidomic evidence that lowering the typical dietary palmitate to oleate ratio in humans decreases the leukocyte production of proinflammatory cytokines and muscle expression of redox-sensitive genes. J. Nutr. Biochem. 2015, 26, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Montes-Borrego, M.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B.; et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J. Clin. Endocrinol. Metab. 2016, 101, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pelaez, S.; Mosele, J.I.; Pizarro, N.; Farras, M.; de la Torre, R.; Subirana, I.; Perez-Cano, F.J.; Castaner, O.; Sola, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Balfego, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martinez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N.; et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.N.; Martins, A.R.; Crisma, A.R.; do Amaral, C.T.L.; Davanso, M.R.; Serdan, T.D.A.; da Cunha de S, R.D.C.; Cruz, M.M.; Alonso-Vale, M.I.C.; Torres, R.N.P.; et al. Combination of a high-fat diet with sweetened condensed milk exacerbates inflammation and insulin resistance induced by each separately in mice. Sci. Rep. 2017, 7, 3937. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; He, X.; Chin, E.L.; Tovar, A.R.; Torres, N.; Slupsky, C.M.; Raybould, H.E. Nopal feeding reduces adiposity, intestinal inflammation and shifts the cecal microbiota and metabolism in high-fat fed rats. PLoS ONE 2017, 12, e0171672. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed]
- Mujico, J.R.; Baccan, G.C.; Gheorghe, A.; Diaz, L.E.; Marcos, A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br. J. Nutr. 2013, 110, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhou, Y.; Chen, S.H.; Zhao, X.L.; Ran, L.; Zeng, X.L.; Wu, Y.; Chen, J.L.; Kang, C.; Shu, F.R.; et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin e2 in nonalcoholic fatty liver disease associated with hyperlipidemia: A randomized clinical trial. PLoS ONE 2015, 10, e0133496. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef] [PubMed]
- Calder, P. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pinel, A.; Pitois, E.; Rigaudiere, J.P.; Jouve, C.; De Saint-Vincent, S.; Laillet, B.; Montaurier, C.; Huertas, A.; Morio, B.; Capel, F. Epa prevents fat mass expansion and metabolic disturbances in mice fed with a western diet. J. Lipid Res. 2016, 57, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, M.J.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J. Nutr. 2015, 145, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Guadagnini, D.; Tsukumo, D.M.L.; Schenka, A.A.; Latuf-Filho, P.; Vassallo, J.; Dias, J.C.; Kubota, L.T.; Carvalheira, J.B.C.; Saad, M.J.A. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 2012. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Hu, S.; Jacoby, J.J.; Liu, J.; Zhang, Y.; Yu, L. The impact of dietary sn-2 palmitic triacylglycerols in combination with docosahexaenoic acid or arachidonic acid on lipid metabolism and host faecal microbiota composition in sprague dawley rats. Food Funct. 2017, 8, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from europe and rural africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Bibbó, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Deol, P.; Evans, J.R.; Dhahbi, J.; Chellappa, K.; Han, D.S.; Spindler, S.; Sladek, F.M. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: Potential role for the liver. PLoS ONE 2015, 10, e0132672. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Papamiltiadous, E.S.; Roberts, S.K.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Salim, A.; Tierney, A.C. A randomised controlled trial of a mediterranean dietary intervention for adults with non alcoholic fatty liver disease (medina): Study protocol. BMC Gastroenterol. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Wah-Kheong, C.; Khean-Lee, G. Epidemiology of a fast emerging disease in the asia-pacific region: Non-alcoholic fatty liver disease. Hepatol. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Baidal, J.A.W.; Lavine, J.E. The intersection of nonalcoholic fatty liver disease and obesity. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Pastori, D.; Loffredo, L.; Angelico, F. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 8341–8350. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (nafld): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Lottenberg, A.M.; Afonso Mda, S.; Lavrador, M.S.; Machado, R.M.; Nakandakare, E.R. The role of dietary fatty acids in the pathology of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Ascher, M.; Gäbele, E.; Hellerbrand, C.; Kob, R.; Bertsch, T.; Bollheimer, L.C. Olive oil attenuates the cholesterol-induced development of nonalcoholic steatohepatitis despite increased insulin resistance in a rodent model. Horm. Metab. Res. 2013, 45, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, W.; Yao, L.; Zhang, X.; Zhang, X.; Ye, C.; Jiang, H.; He, J.; Zhu, Y.; Ai, D. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 2017, 174, 2358–2372. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ratziu, V.; Oren, R. Nutrition and physical activity in nafld: An overview of the epidemiological evidence. World J. Gastroenterol. 2011, 17, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the mediterranean diet: Views from experts around the world. BMC Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Yang, L.H.; Sha, K.H.; Liu, T.G.; Zhang, L.G.; Liu, X.X. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 7008–7013. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Bhatia, L.; Scorletti, E.; Smith, D.E.; Jackson, N.C.; Shojaee-Moradie, F.; Umpleby, M.; Calder, P.C.; Byrne, C.D. Docosahexaenoic acid enrichment in nafld is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: A pilot study. Eur. J. Clin. Nutr. 2017, 71, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vicario, C.; Gonzalez-Periz, A.; Rius, B.; Moran-Salvador, E.; Garcia-Alonso, V.; Lozano, J.J.; Bataller, R.; Cofan, M.; Kang, J.X.; Arroyo, V.; et al. Molecular interplay between delta5/delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 2014, 63, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.K.; Nookaew, I.; Sandberg, A.-S.; Gabrielsson, B.G. Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Frantz, E.D.C.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014, 49, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective effects of various ratios of dha/epa supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis. 2017, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Philbrick, K.A.; Jump, D.B. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a ldlr−/− mouse model of western diet-induced nonalcoholic steatohepatitis. J. Nutr. 2013, 143, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lytle, K.A.; Depner, C.M.; Wong, C.P.; Jump, D.B. Docosahexaenoic acid attenuates western diet-induced hepatic fibrosis in ldlr−/− mice by targeting the tgfbeta-smad3 pathway. J. Lipid Res. 2015, 56, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Hanke, D.; Zahradka, P.; Mohankumar, S.K.; Clark, J.L.; Taylor, C.G. A diet high in alpha-linolenic acid and monounsaturated fatty acids attenuates hepatic steatosis and alters hepatic phospholipid fatty acid profile in diet-induced obese rats. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yuan, F.; Wang, H.; Tian, Y.; He, L.; Shao, Y.; Li, N.; Liu, Z. Perilla oil supplementation ameliorates high-fat/high-cholesterol diet induced nonalcoholic fatty liver disease in rats via enhanced fecal cholesterol and bile acid excretion. BioMed Res. Int. 2016, 2016, 2384561. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Ma, T.; Liaset, B.; Keenan, A.H.; Araujo, P.; Lock, E.J.; Demizieux, L.; Degrace, P.; Frøyland, L.; Kristiansen, K.; et al. Dietary eicosapentaenoic acid supplementation accentuates hepatic triglyceride accumulation in mice with impaired fatty acid oxidation capacity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, A.; Milani, S.; Vizzutti, F.; Delogu, W.; Navari, N.; Novo, E.; Maggiora, M.; Maurino, V.; Laffi, G.; Parola, M.; et al. N-3 polyunsaturated fatty acids worsen inflammation and fibrosis in experimental nonalcoholic steatohepatitis. Liver Int. 2014, 34, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Ruiz, E.; Varela, L.M.; Luque, A.; Berna, G.; Cahuana, G.; Martinez-Force, E.; Gallego-Duran, R.; Soria, B.; de Roos, B.; Romero Gomez, M.; et al. An extra virgin olive oil rich diet intervention ameliorates the nonalcoholic steatohepatitis induced by a high-fat “western-type” diet in mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Zhang, W.; Wang, H.; Ong, K.T.; Woo, S.L.; Walzem, R.L.; Mashek, D.G.; et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012, 7, e39286. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yang, L.; van Rooijen, N.; Brenner, D.A.; Ohnishi, H.; Seki, E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013, 57, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Böhm, T.; Berger, H.; Nejabat, M.; Riegler, T.; Kellner, F.; Kuttke, M.; Sagmeister, S.; Bazanella, M.; Stolze, K.; Daryabeigi, A.; et al. Food-derived peroxidized fatty acids may trigger hepatic inflammation: A novel hypothesis to explain steatohepatitis. J. Hepatol. 2013, 59, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Traber, M.G.; Bobe, G.; Kensicki, E.; Bohren, K.M.; Milne, G.; Jump, D.B. A metabolomic analysis of omega-3 fatty acid-mediated attenuation of western diet-induced nonalcoholic steatohepatitis in ldlr−/− mice. PLoS ONE 2013, 8, e83756. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Steve, M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2009, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Brenner, D.A. Toll-like receptors and adaptor molecules in liver disease: Update. Hepatology 2008, 48, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Csak, T.; Ganz, M.; Pespisa, J.; Kodys, K.; Dolganiuc, A.; Szabo, G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011, 54, 133–144. [Google Scholar] [CrossRef] [PubMed]

| Host | Fatty Acid Composition | Glycaemia-Related Effects in Obesity | References |
|---|---|---|---|
| Humans | Diets with 63% SFA (42% palmitic, 29% MUFA, 4% PUFA) | Increased glycaemia (3.70%) Increased insulin (25%) | [22] |
| Hypertensive women with DM II | (1) 1.5 g fish oil (21.9% EPA, 14.1% DHA) (2) 2.5 g fish oil (21.9% EPA, 14.1% DHA) (3) Control group. | Glucose, mg/dL; glycated hemoglobin, %; insulin, µU/mL and HOMA-IR without changes. | [26] |
| Diabetics and nondiabetics individuals | (1) 300 g of vegetables and 25 mL of PUFA-rich plants (61.8% linoleic, 11.5% linolenic, and 16.4% of oleic fatty acid) per day | Reduction of HbA1c (hemoglobin A1c) (%) after 4 and 8 weeks | [27] |
| Subjects with early-stage DM II or metabolic syndrome | Individuals received corn oil (CO); a combination of borage [Borago officinalis L.] and echium oil [Echium plantagineum L.] (BO) or fish oil (FO): 9 CO capsules, 10 BO capsules (3 borage and 7 echium), or 9 FO capsules | Statistically significant increase in insulin and reduction in HbA1c of FO group. | [28] |
| DM II subjects | Supplementation of 3 g/day of ALA or placebo for 60 days | ALA group improved IS corrected for FFM (M/FFM)—Insulin sensitivity corrected for fat-free mass. | [29] |
| DM II subjects | (1) High-carbohydrate/high-fiber/low-glycemic index diet (CHO/fiber group) (2) High-MUFA diet (MUFA group) (3) High-carbohydrate/high-fiber/low-glycemic index diet plus physical activity program (CHO/fiber + Exercise group) (4) High-MUFA diet plus physical activity program (MUFA + Ex group). | Reduction of HbA1c levels in the MUFA group. | [30] |
| Human clinical trials: obese children | Supplementation of CLA (3 g/day) with 50:50 isomers c9, t11, and t10, c12 or placebo (1 g/day) 3 times per day for 16 weeks | Significant improvement in insulin, fasting insulinemia, and HOMA-IR in CLA group. | [31] |
| Host | Fatty Acid Composition | Glycaemia-Related Effects in Obesity | References |
|---|---|---|---|
| In vitro insulin resistance at cellular level from thoracic aorta arteries of three 8-week-old wild-type male mice | Cell lines were cultured with high glucose and were serum-starved for insulin signaling and relatives free fatty acids (palmitate or oleate) | Oleate treatment for 2 h did not produce insulin resistance. Palmitate significantly induced insulin resistance for 18 h. | [36] |
| C57BL/6 male mice | SFA High Fat Diet (HFD) with 45% palmitic acid; MUFA-HFD (45% oleic acid), and a standard chow as a control group (5.2% fat: 0.9% SFA, 1.3% MUFA, and 3.4% PUFA) | Lower fast glucose, insulin concentrations and insulin secretion in MUFA-HFD group compared to the SFA-HFD group. | [40] |
| Hypertriglyceridemia-induced dyslipidemia rats | High sucrose diet supplemented with either sunflower oil or Conjugated Linoleic Acid (CLA) (2 g/100 g diet) | Decrease in glucose and insulin (mmol/L) in CLA supplemented group. | [39] |
| Diet-induced IR rat model | Supplementation of fish oil (n-3 PUFA), sunflower oil (n-6 PUFA), and high oleic sunflower oil (n-9 MUFA) | Reduction of HOMA-IR in n-3 PUFA. | [43] |
| Host | Fatty Acid Composition | Effects | References |
|---|---|---|---|
| Humans with hypertriglyceridemia | n-3 PUFA (2,3 and 4 g of fish oil) | Reduction in VLDL, TG, non-HDL, LDL and Apo-B | [62] |
| Humans: Hemodialysis Patients | 2 capsules of EPA and 1.28 g DHA/day | TG, TC, and LDL (no differences) EPA/DHA and placebo. Increase in HDL. | [65] |
| Humans | 2 capsules of 900 mg/day containing EPA and DHA | Increase in HDL, reduction in LDL and TG. Improvement Protein C reactive levels. | [66] |
| Humans | 4 capsules of 1 g/day containing EPA and DHA for 6 months | Reduction in TG, increase in HDL. No difference in TC and LDL. | [67] |
| Humans | 4 different foods enriched with 3 rich-n-3-PUFA oils | Increase in HDL. LDL—no differences. | [68] |
| Host | Fatty Acid Composition | Effects | References |
|---|---|---|---|
| Wistar rats | Three diets and a control group (7% fat): CG (Saturated fatty acid); SO (Sesame oil—oleic and linoleic fatty acid); FO (Flaxseed oil—alfa-linolenic fatty acid), and SFO (flaxseed and sesame oil) | Increased levels of total cholesterol, HDL, VLDL, and TAG in CG and SO groups. Reduction in levels in non-HDL and LDL for SFO group. | [76] |
| Wistar rats | 6 groups: control (AIN-93G—7% soy oil); extra virgin oil (OO-C) (7% soy oil and 13% extra virgin); sunflower oil (HOSO) (7% soy oil and 13% sunflower oil); Atherogenic diet (AT), (rich-SFAs (12.3 g %) and cholesterol (4 g %); Experimental diets were: OO and HOSO (11.82% and 12.9 g % MUFA and 4% cholesterol). | HOSO: Increase in TC and non-HDL, HDL diminished and decrease in TG in comparison to AT. OO: Reduced TC and non-HDL. | [79] |
| Wistar rats | 4 groups over 5 weeks: Extra virgin olive oil group (OO) (SFA 12.0%, MUFA 81.9%, PUFA 6.10%), sunflower group (HOSO) (SFA 7.82%, MUFA 87.11%, PUFA 4.75%), sunflower oil and phytosterols group (HOSO-F) (1% phytosterols); sunflower oil and n-3-PUFA (HOSO-P) (6.5% fish oil). | HOSO: Increase in TC and non-HDL and reduction in HDL; HOSO-P and HOSO-F: Decrease in TC, non-HDL and TAG and increase in HDL in comparison to the OO group. | [80] |
| Wistar rats | High fat (HF) diets enriched in saturated fatty acids (SFAs); MUFA (oleic acid); PUFA n-6 and PUFA n-3. | TG decreased in MUFA and PUFA n-6 just at first day; Reduction in TG levels with a longer time feeding (21 days) | [81] |
| Host | Fatty Acid Composition of the Experiment | Microbiota | Inflammatory Process | References |
|---|---|---|---|---|
| Adults individuals | Control group (28.4% fat, of which 5.3% was palmitic fatty acid and 15.9% was oleic fatty acid); High fat (40.4% fat, of which 16% was palmitic fatty acid and 16.2% was oleic fatty acid); High fat (40.4% fat, of which 2.4% was palmitic fatty acid and 28.8% was oleic fatty acid) | Not observed | ↓ IL-1β, IL-10, IL-18, and TNF-α ↑ IL-1β, IL-10, IL-18, and TNF-α | [93] |
| Obese children and adolescents (BMI >95th percentile for sex and age) | Therapeutic protocol: ↓ Fat ↓ Sugar ↑ Fibers | Not observed | ↓ IFN-γ, IL-12A, IL-18, TNF-α, IL-6, IL-1β. | [90] |
| Adult individuals | Control group (20% fat/olive oil—MUFA) High fat with n-3 PUFA (35% fat with fish oil) High fat with n-6 PUFA (35% fat and grapeseed oil) High Fat with SFA (35% fat and coconut oil) | Not observed | ↓ endotoxins postprandial ↑ endotoxins postprandial | [91] |
| Obese individuals | Mediterranean Diet (35% fat, 22% monounsaturated) Low-fat, high-complex carbohydrate diet diet (28% fat, 12% monounsaturated) | ↑ Roseburia and Oscillospita and ↓ Prevotella ↑ Prevotella, ↓ Roseburia and ↑ F. prausnitzii | Not obeserved | [94] |
| Metabolic syndrome “at-risk” population | HS: High saturated fatty acids diet High monounsaturated fat (MUFA)/high glycemic index (GI) (HM/HGI) High MUFA/low GI (HM/LGI) High carbohydrate (CHO)/high GI (HC/HGI) High CHO/low GI (HC/LGI) | ↑ Bifidobacterium and Bacteroidetes | [95] | |
| Hypercholesterolemic individuals | Virgin olive oil (OO) naturally containing 80 mg of PC/kg, (VOO) Phenolic compound (PC) enriched virgin olive oil containing 500 mg PC/kg, from OO (FVOO) PC-enriched virgin olive oil containing a mixture of 500 mg PC/kg from OO and thyme 1:1 (FVOOT) | ↑ Bifidobacterium, Parascardovia denticolens and Roseburia | [96] | |
| DM 2 subjects | Control group Sardine group (SG) | ↓ Firmicutes/Bacteroidetes ↓ Firmicutes/Bacteroidetes and ↓ bacteroidetes/prevotella | ↑ TNF-α ↑ Adiponectin | [97] |
| Host | Fatty Acid Composition of the Experiment | Microbiota | Inflammatory Process | References |
|---|---|---|---|---|
| Female rats | Control group (10% kcal fat), high Fat (60% kcal fat, of which 34% was SFA) | ↑ Firmicutes and ↓ Bacteroidetes | ↑ Inflammatory citokines | [100] |
| Female mice | Control group (12.6% fat) High fat (60.3% fat) High fat with oleic fatty acid High Fat with n-3 PUFA (EPA and DHA) | ↑ Firmicutes and Enterobacteria, ↓ Bifidobacteria ↓ Firmicutes and ↑ Bifidobacteria ↑ Firmicutes | Not observed | [101] |
| Male rats | Control group with palmitic fatty acid Palmitic fatty acid with DHA Palmitic fatty acid with ALA | ↑ Lactobacillus ↑ Lactobacillus and Allobaculum, ↓ Proteobacteria | Not observed | [102] |
| Elderly male rats | Normolipid diet (12% fat) High Fat (43% fat) | ↓ Firmicutes ↓ Lactobacillus | Not observed | [103] |
| Male rats | Placebo (10% skimmed milk) High Fat with placebo Placebo with 1 × 109 CFU. B. pseudocatenalatum High Fat diet with 1 × 109 CFU. B. pseudocatenalatum | ↑ Firmicutes (65%) and Bacteroidetes (31%) ↑ Firmicutes, ↓ Bacteroidetes, ↑ Proteobacteria ↑ Firmicutes (66%) and Bacteroidetes (31%) ↑ Firmicutes, ↓ Bacteroidetes | ↑ CD8+/CD4+, ↑ TNF-α, MCP-1, IL-10, IL-17A, IP-10, IL-6, ↑ LPS ↓ CD8+/CD4+, ↓ TNF-α, MCP-1, IP-10, 1L-17A, IL-6, ↓ LPS | [90] |
| Male rats | Normolipid diet (10% fat) with Nopal (4% fiber) High fat (46% fat) with Nopal (4% fiber) | ↑ Firmicutes ↑ Bacteroidetes | ↑ IL-6 ↓ IL-6, ↓ in adipocyte size | [99] |
| Male rats | Control group Control group with high sugar (HS) High fat High fat with HS | Not observed | ↑ size of adipocytes and hepatocytes ↑ TNF-α ↑ IL-6, ↑ IL-1 β | [98] |
| Host | Fatty Acid Composition | Effects | References |
|---|---|---|---|
| Human Clinical Trial: Adults | - Mediterranean diet: olive oil, vegetables, legumes, nuts, fruits, whole grains, fish and seafood, moderate wine - Low-fat-high carbohydrate diet (LF/HCD) Duration: 6 weeks (6-week wash-out period in-between) | - Weight loss was not observed between the two diets - Reduced hepatic steatosis - Improved insulin sensitivity (HOMA-IR) - No differences in peripheral insulin resistance | [126] |
| Human Clinical Trial: Adults | - Mediterranean diet and Physical activity Duration: 6 months | - Improved BMI, waist circumference, waist-to-rip ratio, ALT, GGT, serum glucose, total cholesterol/HDL, LDL/HDL, TG/HDL, HOMA, NAFLD score | [120] |
| Human Clinical Trials: Adults | n-3 PUFAs - (50 mL of PUFA with 1:1-DHA: EPA into daily diet) Duration: 6 months | - Reduced ALT and AST levels - Reduced triacylglycerol (TG), total cholesterol (TC) levels - Reduced systemic inflammatory markers: C-reactive protein (PCR) - Reduced pro-oxidant factors: malondialdehyde (MDA) - Reduced fibrosis parameters: type IV collagen and pro-collagen type III pro-peptide | [127] |
| Human Clinical Trials: Adults | n-3 PUFAs - 2 capsules fish oil 2 times per day (182 mg EPA and 129 mg DHA) - 2 capsules corn oil 2 times per day (without EPA and DHA) Duration: 3 months | - Reduced TG, TC, apolipoprotein B, glucose, ALT, GGT. - Increased serum adiponectin levels. - Reduced NAFLD biomarkers: fibroblast factor growth 21 (FGF-21) and CK18 fragment M30 (CK18-M30). - Reduced pro-inflammatory cytokines: tumor necrosis factor-α (TNF-α), leukotrienes 4, and prostaglandin E2. - Corn oil increased creatinine serum levels, but without other metabolic effects. | [102] |
| Human Clinical Trials: Adults | n3-PUFAs 4 g/day EPA and DHA - Placebo Duration: 15–18 months | - Erythrocyte DHA enrichment ≥2%: no changes in fat liver content. - Fat liver reduction: decrease in hepatic DNL with concomitant increase hepatic FA oxidation and hepatic insulin sensitivity. | [128] |
| Host | Fatty Acid Composition | Effects | References |
|---|---|---|---|
| Mice and In vitro | n-3 PUFAS - HFD-fed mice - n-3 PUFA-enriched HFD (17,18-EEQ, 5-HEPE, 9-HEPE (efficient components of HEPEs and EEQs metabolites) Duration: 4 days - In vitro: Primary hepatocytes and peritoneal macrophages | Mice: Reduced macrophage infiltration in adipose tissue - Reduced pro-inflammatory cytokines (IL-6, MCP-1 and TNF-α) in plasma content In vitro: activation of pro-inflammatory cytokines as well as activation of JNK pathway by palmitate in macrophages were reduced through the mixture of 17,18-EEQ, 5-HEPE, 9-HEPE | [123] |
| Mice | Corn oil and n3-PUFAs - Corn-oil based HFD - n3-PUFA DHA/EPA-enriched diet Duration: 12 weeks | - The quality of the diet (n3-PUFA) could modulate liver transcriptoma: - corn oil based HFD: modulate PPAR-related gene expression and have induced PPAR-γ gene signatures - DHA/EPA-enriched diet: induced genes known to be regulated by PPAR-α | [130] |
| Mice | n3-PUFAs - HFD-fed mice - n3 PUFA-enriched HFD Duration: 8 weeks | - n3-PUFA-enriched HFD: without obesity, liver damage, hypertriglyceridemia, hepatic insulin resistance, steatosis - Improved hepatic glucose output - Reduced expression of genes related to lipogenesis: SREBP-1C and FAS - Improved inflammatory markers: increase adiponectin levels - Increased beta oxidation with increased expression of PPARα and PPAR-α target and CPT-1 | [131] |
| Mice | n3-PUFAs - HFD-fed mice - DHA/EPA supplementation in HFD (different ratios 1:2, 1:1 and 2:1) Duration: 11 weeks | - Best suggestion: Ratio 1:2 - Increase HDL/C levels - Reduced ALT, AST, MDA levels and increased glutathione (GSH) levels - Reduced the expression of lipid metabolism genes: SREPB-1C, SCD-1, ACC-1 and PPAR-γ - Lowered expression of proteins expression levels c-Jun and c-Fos - Weakened activation of Ap-1 - Reduced inflammatory cytokines (IL-6 and IL-1β) | [132] |
| Mice | MUFA and n3-PUFAs - Western diet supplemented with olive oil (OO) (WD + OO), - Westerm diet supplemented with EPA (WD + EPA) - Western diet supplemented with DHA (WD + DHA) - Western diet supplemented with DHA + EPA (WD + DHA/EPA) Duration: 16 weeks | - WD + OO: severe NASH phenotype accompanied with inflammation, oxidative stress and fibrosis - WD + DHA/EPA: attenuated ALT and AST levels - WD + DHA: - Reduced cell surface markers for Kupffer cells and macrophages in liver Clec4f; Clec10a; CD68; and F4/80) - Diminished inflammatory markers like IL-1β, TNF-α, TLR4, TLR-9 and genes involved in TLR pathway Cd-14 and MyD88 - Blocked WD-induced accumulation of nuclear factor κ beta (NFκB) in hepatic nuclei - Reduce oxidative stress (NADPH oxidase subunits Nox2, p22phox, p40phox, p47phox, p67phox) - Diminished Procol1α1 - Reduced cytokine TGF-β1 | [133] |
| Mice and In vitro | MUFA and n3-PUFAs - Western diet supplemented with olive oil (OO) (WD + OO), - Westerm diet supplemented with EPA (WD + EPA) - Western diet supplemented with DHA (WD + DHA) - Western diet supplemented with DHA + EPA (WD + DHA/EPA) Duration: 16 weeks In vitro: Human LX2 stellate cells treated with DHA | WD + DHA: No increase in hepatic nuclear abundance (Smad 3) - WD+OO and WD+EPA: Increased Smad3 expression. In vitro: Human LX2 stellate cells: - Blocked TGF-β mediated induction of Col1A1 | [134] |
| Rats | Canola Oil, Soybean Oil, Safflower Oil, Lard - High oleic canola oil (HOC) - Conventional canola oil (C) - Conventional canola oil/flax oil blend (C/F) (3:1 ratio) - High linoleic safflower oil (SF) - Soybean oil (SB) - Lard and soybean oil (L) - Weight-matched group fed lard and soybean oil (WM) Duration: 12 weeks. | - C/F group: - Attenuated hepatic stetatosis—Lower concentration of fat liver - Altered hepatic phospholipids fatty acid profile by increasing EPA and DHA. - HOC, C and C/F groups: - Gained the least of body weight: lowest weight gain without differences in adiposity | [135] |
| Rats | n3-PUFAs Perilla oil - High-fat diet/high-cholesterol diet (HFD/HC) - Perilla oil-enriched diet (POH) | - POH group: - Improved HFD-induced hyperlipidemia (TG, CT and LDL) - Reduced hepatic steatosis - Diminished activity of ALT and AST enzymes - Reduced hepatic inflammatory infiltration around portal area - Rescued HFD-induced hepatic fibrosis - Abrogated downregulation of ABCG 5 and ABCG 8 - Increased the expression of CYP2A1 and CYP27A1 | [136] |
| Mice | n3-PUFAs EPA - HFD-fed mice - HFD-enriched 3% EPA + 500 mg milidronate/kg/day - HFD-enriched 3% EPA Duration: 10 days | - HFD-enriched 3%: - Accentuated hepatic triglyceride accumulation. - HFD-enriched 3% EPA + 500 mg milidronate/kg/day: - Exacerbation of milindronate-induced triglyceride accumulation - EPA decreased the milidronate-induced mRNA expression of inflammatory genes: MPEG1, COX 2, CD68, F4/80 - Increased GRP120 | [137] |
| Mice | n3-PUFAs and n-9 MUFAs - Methionine and choline deficient (MCD) diet - MCD-enriched diet n-3 PUFA + n-9 MUFA (EPA/DHA 25 mg + OO 75 mg) (MCD/n-3) - MCD-enriched diet n-9 MUFA alone (OO 100 mg) (MCD/OO) two times a week by intragastric gavage. Duration: 8 weeks | - MCD/n-3 group: higher levels of ALT, severe scores of inflammation - Increased intrahepatic expression of inflammatory markers: TNF-α and CCL2 - Increased expression of profibrogenic genes: TGF-β1 - Increased tissue inhibitor of metalloproteinase (TIMP-1) - Higher portal pressure | [138] |
| Mice | n-9 MUFA - Standard chow diet (SCD) - HFD based on lard (HFD—49 energy % of fat) Duration: 12 weeks HFD-fed mice were divided in four groups: - Unchanged HFD-L (HFD-L) - HFD based on EVOO (HFD-EVOO) - HFD based on EVOO rich in phenols (HFD-OL with same percentage of fat) - R (reversion, LFD) Duration: 24 weeks | - HFD-EVOO: - Reduced body weight - Improved plasma lipid profile - Reduced pro-inflammatory citokynes in epididimal adipose tissue: IFN-γ, IL-6, leptin and macrophage infiltration - Diminished NAFLD activity (NAS) score - Reduced hepatic adiponutrin (Pnpal3) - Increased Cd36 gene | [139] |
| Mice and In vitro | Palmitoleate n-7 MUFA - LFD - LFD + Palmitoleate LFD + Oleate | - LFD+Palmitoleate: -Improved systemic insulin-sensitivity - Induced hepatic steatosis Improved insulin signaling in liver: insulin-stimulates Akt (Ser 473) phosphorylation - Reduced phosphorylation of NFκB p65 (Ser468) - Reduced expression of IL-6 and TNF-α. In vitro: hepatocytes and RAW macrophaged+palmitoleate: - Increased fat deposition’ - Stimulated FAS expression - Activated SREBP-1c - Decreased inflammation: NFκB p65 Ser 68, TNF-α, IL-6 in both hepatocytes and RAW macrophages. | [140] |
| In vitro | Palmitic acid (PA) SAFs In vitro: Kupffer Cells and stellate cells stimulated with TLR2 and palmitic acid | In vitro (Kupffer cells) were more important than HSC in TLR2-mediated progression of NASH - TLR 2 ligand increased NOD3 (inflammasome) in Kuppfer cells. - PA together with TLR2 ligand: Induced caspase-1 activation in Kupffer cells - Released IL-1β and IL-1α in Kuppfer cells | [141] |
| Rats and In vitro | Corn Oil - peroxidized Fat - Corn oil peroxidized oil (PO) - Unperoxidized FA (OIL) - Tap water (WA) gavage Duration: 6 days. | - PO group: - Increased pro-oxidant state NOS-2, NO-formation and pronounced lipid peroxidation in liver - Decrease in α- and γ-tocopherol in liver. - Increased inflammatory markers: TNFα, COX-2, IL-1β and macrophage markers cd68 and cd 163 in the liver In vitro: hepatocytes, endothelial and Kupffer cells and incubated with peroxidized linoleic acid: more pronounced in Kupffer cells: - Augmented the secretion of TNF-α, mRNA expression of TNF-α, NOS-2, COX-2 - Increased p38MAPK phosphorylation | [142] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; De Cássia Freitas, K.; De Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. https://doi.org/10.3390/nu9101158
Silva Figueiredo P, Carla Inada A, Marcelino G, Maiara Lopes Cardozo C, De Cássia Freitas K, De Cássia Avellaneda Guimarães R, Pereira de Castro A, Aragão do Nascimento V, Aiko Hiane P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients. 2017; 9(10):1158. https://doi.org/10.3390/nu9101158
Chicago/Turabian StyleSilva Figueiredo, Priscila, Aline Carla Inada, Gabriela Marcelino, Carla Maiara Lopes Cardozo, Karine De Cássia Freitas, Rita De Cássia Avellaneda Guimarães, Alinne Pereira de Castro, Valter Aragão do Nascimento, and Priscila Aiko Hiane. 2017. "Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders" Nutrients 9, no. 10: 1158. https://doi.org/10.3390/nu9101158
APA StyleSilva Figueiredo, P., Carla Inada, A., Marcelino, G., Maiara Lopes Cardozo, C., De Cássia Freitas, K., De Cássia Avellaneda Guimarães, R., Pereira de Castro, A., Aragão do Nascimento, V., & Aiko Hiane, P. (2017). Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients, 9(10), 1158. https://doi.org/10.3390/nu9101158