1. Introduction
Obesity is a condition in which a person has an abnormally high and unhealthy proportion of body fat. Obesity is a major risk factor for many metabolic disorders, including hyperlipidemia, diabetes mellitus, atherosclerosis, hypertension, and cardiovascular disease [
1]. Physiologically, obesity is associated with increased levels of adipocytes and an increase in adipocyte volume. Although accumulated intracellular triglycerides can be broken down by exercise or diet, obesity caused by increased fat-cell size and the number is difficult to treat, as the fat cells must be destroyed or removed.
Adipogenesis is a multi-step process involving a cascade of transcription factors and adipocyte-specific gene expression leading to adipocyte development. Lipid accumulation reflects the process of adipogenesis, which is regulated by genetic and growth factors [
2,
3]. Adipogenesis is a differentiation process by which preadipocyte cells undergo terminal differentiation to mature adipocytes. CCAAT/enhancer binding protein-δ (C/EBPδ) and CCAAT/enhancer binding protein-β (C/EBPβ) are rapidly and transiently expressed after the hormonal induction of differentiation [
4,
5]. These genes act synergistically to promote the expression of CCAAT/enhancer binding protein-α (C/EPBα) and peroximal proliferator-activated receptor-γ (PPARγ), which are the master adipogenic transcription factors [
6,
7]. After differentiation, adipocytes regulate lipid metabolism through lipogenic proteins such as fatty acid synthase (FAS) and aP2 [
7].
Insulin and Akt signalling modulates adipose tissue growth and adipogenesis [
8]. Insulin stimulates glucose and free fatty acid uptake, inhibits lipolysis, and stimulates de novo fatty acid synthesis in adipocytes. The Ser/Thr kinase Akt plays an essential role in adipocyte differentiation. Mouse embryonic fibroblasts (MEFs) lacking Akt display an inability to differentiate into adipocytes [
9], and an RNAi-mediated decrease in Akt was found to block the differentiation of 3T3-L1 cells [
10]. Glycogen synthase kinase-3β (GSK-3β), which controls glycogen and protein synthesis among many other cellular processes, was one of the first described physiological targets of Akt [
11].
Recently, the medical use of natural plant products could provide more effective and less expensive medications for people than ever before.
Artemisia annua L., also known as annual wormwood (AW), is a common type of wormwood that belongs to the family Asteraceae. Annual wormwood leaves (AWL) have been used for many centuries in traditional medicine in Asia in the treatment of febrile diseases and malaria. Among major components such as monoterpenes, camphor, and Artemisia ketone in
Artemisia annua L., artemisinin, which has a critical role in anti-oxidant and anti-inflammatory with formula C
15H
22O
15 and characterized by structural features, contains a peroxide bridge (C-O-O-C) [
12]. As an important biological functional, artemisinin is known to have anti-bacterial, anti-fungal, anti-leishmanial, anti-oxidant, anti-tumour, and anti-inflammatory activities [
13,
14,
15]. In particular, artemisinic acid isolated from AW inhibited adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells [
16].
Annual wormwood is associated with many health benefits. However, it remains unknown how AWL promotes an anti-obesity effect in 3T3-L1 adipocytes and high fat diet (HFD)-induced obese rats. In the present study, the effect of AWL extracts on adipocyte differentiation in 3T3-L1 cells were investigated by measuring the accumulation of intracellular droplets of triglyceride as well as the expression levels of several adipogenesis-related genes. Moreover, in order to understand the specific mechanisms of these effects, we examined whether Akt and GSK3β activation is critical for the anti-adipogenic functions of AWL. We further evaluate anti-obesity effects of AWL in obese rats fed high-fat diets (HFDs).
2. Materials and Methods
2.1. Preparation of Annual Wormwood Leaf (AWL) Extracts
Fresh leaves of annual wormwood (AW) were collected immediately after harvesting in May 2016 at Jinju, Gyeongnam (Animal Bio-Resources Bank, Gyeongnam, Korea). Annual wormwood leaves (AWL) were authenticated by Professor T. H. Kim in the Department of Food Science and Biotechnology, Daegu University, Korea. Fresh samples of annual wormwood leaves were prepared by alcohol extraction. The leaves were chopped after washing with running water, dried in oven at 40 °C for 2 days followed by grinding to a powder. The AWL (30 g) powder was then suspended in an 80% (v/v) ethanol solution using a mixer, followed by extraction of the samples for 3 days with vigorous shaking at room temperature and filtering through Whatman No. 1 filter paper. The ethanolic extracts of AWL were concentrated using rotary-vacuum evaporation at 50 °C and then freeze-dried.
2.2. Cell Culture
Mouse 3T3-L1 preadipocytes were purchased from the Korean Cell Line Bank (Seoul, Korea) and cultured as described elsewhere [
17]. In brief, cells were cultured in Dulbecco’s Modified Eagle High-glucose Medium (DMEM) supplemented with 10% calf serum at 37 °C in a humidified atmosphere of 5% CO
2. At 1 day postconfluence (designated “day 0”), cell differentiation was induced with a mixture (DMI) of 0.5 mM 3-isobutyl-1-methylxanthine, 100 μM indomethacin, 0.25 μM dexamethasone and 167 nM insulin in DMEM containing 10% FBS. The 3-isobutyl-1-methylxanthine (MIX), dexamethasone (DEX), indomethacin, and Oil Red O were obtained from Sigma-Aldrich (St. Louis, MO, USA). The medium was changed every 2 days. AWLs were added to the culture medium of the adipocytes on day 0. The cells were treated with 0, 25, or 100 μg/mL of AW extracts every day. After treatment with AWL for 4 and 7 days, the 3T3-L1 adipocytes were lysed for Western blot analysis. To analyse cell viability, the cytotoxicity of the AWL was evaluated using 3-(4, 5-demethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT).
2.3. Oil Red O Staining
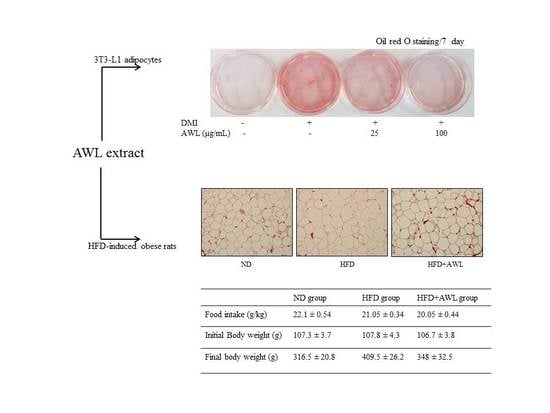
The cellular lipid content was assessed by Oil Red O staining (Sigma, St. Louis, MO, USA). Cells were treated either with AWL extracts (25 μg/mL or 100 μg/mL) or vehicles in the differentiation medium for days 0–7 of adipogenesis. On days 4 or 7, cells were stained with Oil Red O. For Oil Red O staining, cells were washed gently with phosphate-buffered saline (PBS), and stained with filtered Oil Red O solution (60% isopropanol and 40% water) for 30 min. After staining the lipid droplets red, the Oil Red O staining solution was removed and the plates were rinsed with water and dried. After 3 washes with PBS, cells were photographed with a 12-megapixel digital camera (Canon, Tokyo, Japan).
2.4. Measurement of Triglyceride Content
Cellular triglyceride content was measured using a commercial Triglyceride Assay Kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions. Adipocytes differentiated for 4 or 7 days were treated with the AWL at concentrations of 0, 25 and 100 μg/mL in 6-well plates. To analyse the content of cellular triglycerides, cells were washed with PBS and then scraped into 200 μL PBS and homogenized by sonication for 1 min. The lysates were assayed for total triglycerides using the assay kits.
2.5. RT-PCR
RNA was isolated from 3T3-L1 adipocytes or epididymal adipocyte tissue using the RNeasy plus Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Two micrograms of total RNA was used for first-strand cDNA synthesis with oligo (deoxythymidine) primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The target cDNA was amplified using the following primers: C/EBPβ, 5′-GACTACGCAACACACGTGTAACT-3′ and 5′-CAAAACCAAAAACATCAACAACCC-3′; PPARγ, 5′-TTTTCAAGGGTGCCAGTTTC-3′ and 5′-AATCCTTGGCCCTCTGAGAT-3′; C/EBPα, 5′-TTACAACAGGCCAGGTTTCC-3′ and 5′-GGCTGGCGACATACAGATCA-3′; aP2, 5′-TGATGCCTTTGTGGGAACCT-3′ and 5′-GCAAAGCCCACTCCCACTT-3′; ACC, 5′-GAATCTCCTGGTGACAATGCTTATT-3′ and 5′-GGTCTTGCTGAGTTGGGTTAGC-3′; FAS, 5′-TGTGAGTGGTTCAGAGGCAT-3′ and 5′-TTCTGTAGTGCCAGCAAGCR-3′; β-actin (control), 5′-GACAACGGCTCCGGCATGTGCAAAG-3′ and 5′-TTCACGGTTGGCCTTAGGGTTCAG-3′. The amplification cycles included denaturation at 95 °C for 50 s, annealing at 55 °C for 1 min and elongation at 72 °C for 50 s. After 30 cycles, the PCR products were separated by electrophoresis on a 1.5% agarose gel for 30 min at 100 V. The gene mRNA levels were normalized using β-actin. The gels were stained with 1 mg/mL ethidium bromide and visualized with UV light using Bio-Rad Gel Doc image analysis software (Bio-Rad Laboratories Inc., Hercules, CA, USA).
2.6. Western Blot Analysis
Western blotting was performed according to standard procedures. Briefly, cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 8.0), 0.4% Nonidet P-40, 120 mM NaCl, 1.5 mM MgCl2, 0.1% sodium dodecyl sulfate (SDS), 2 mM phenylmethylsulfonyl fluoride, 80 μg/mL leupeptin, 3 mM NaF and 1 mM Dithiothreitol (DTT). Cell lysates (50 μg protein) were separated by 10% SDS-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane (Amersham Pharmacia, Little Chalfont, England, UK), blocked with 5% skim milk and hybridized with primary antibodies. PPARγ, C/EBPβ, C/EBPα, aP2, Akt, and GSK3β antibody were from Cell Signaling (Danvers, MA, USA) and the monoclonal β-actin antibody was from Chemicon (Temecula, California, USA). Horseradish peroxidase (HRP)-labelled mouse anti-rabbit IgG were from Jackson ImmunoResearch (West Grove, PA, USA). The Chemiluminescence Kit was from Pierce (Rockford, IL, USA). After incubation with horseradish-peroxidase-conjugated secondary antibody at room temperature, immunoreactive proteins were detected using a chemiluminescent ECL Assay Kit (Amersham Pharmacia, Little Chalfont, England, UK) according to the manufacturer’s instructions.
2.7. Animals and Diets
Four-week-old Sprague–Dawley male rats were purchased from Central Lab Animal Inc. (Seoul, Korea). All animal experiments were performed following the ethical guidelines set out by the Gyeongsang National University’s institutional animal care and with the approval of the Animal Care and Use committee of Gyeongsang National University (Approval Number: GNU-160912-R0032). The experiments began after acclimating the animals for 7 days under constant conditions of temperature (22 °C), humidity (55%), and light (12 h cycle dark/light) in polycarbonate cages. The animals were randomly divided into three groups (n = 10) and fed the normal or experimental diets for 5 weeks as follows: (1) a normal diet group (ND, n = 10); (2) a high-fat diet group (HFD, n = 10); (3) a AWL group (HFD + AWL 150 mg/kg BW, n = 10). Rats in the ND group were fed a normal diet (#55VXT0038, Samyang Co., Seoul, Korea). Feeding rats a high-fat diet produced obese rats, and rats in the HFD groups were fed an HFD based on a commercial diet (rodent diet with 60% kcal fat, Research Diet, Seoul, Korea). The animals were allowed free access to food and water for five weeks. Food intake was measured daily, and the rats were weighed twice per week. At the end of the experiment period, rats were sacrificed after 12 h of fasting.
2.8. Biochemical Analysis
Whole blood samples were centrifuged in a tube containing heparin as anti-coagulant, and isolated serum was used for analysis of triglyceride (TG), total-cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-C). After centrifugation, the organic layer was removed and dried. The resulting pellet was dissolved in phosphate-buffered saline containing 1% Triton X-100, and the triglyceride content was determined using a commercially available Enzymatic Reagent Kit (Asan phams, Co., Hwaseon-si, Korea). The concentrations of total-cholesterol (TC) and high-density lipoprotein-cholesterol (HDL-C) were assayed enzymatically using the commercial kits (Asan phams, Co., Korea).
2.9. Histological Analysis
Epididymal fat tissues were removed and fixed in 10% neutral-buffered formalin. The fat pads were subsequently embedded in paraffin, sectioned into 5 μm sections (Leica, Wetzlar, Germany), and stained with haematoxylin-eosin for microscopic assessment (Olympus, Tokyo, Japan). Three different cross-sectional areas and their corresponding cell populations were assessed using an image analysis program (Image-Pro Plus Version 6.0, Rockville, MD, USA).
2.10. Statistical Analysis
Each experiment was performed at least three times. The data are expressed as the mean ± SD. One-way ANOVA and the Duncan’s multiple tests were used to determine the significant differences between the treatment groups. A p-value < 0.05 was considered statistically significant.
4. Discussion
Obesity is a risk factor for multifaceted metabolic syndromes including Hyperlipidaemia, Type II Diabetes, and Cardiovascular Disease (CVD) [
1,
2]. To date, considerable amount of advancement on target discovery using metabolomics platform with various analytical technologies has been made in the field of metabolic disorder; this includes obesity. Compelling evidence supports that natural substances and herb compounds have focused on prevention and treatment of obesity to achieve a healthy lifestyle. Ongoing high-throughput drug screening in biomedicine leads to a major bottleneck of synthetic chemistry strategies-driven several medications. This reflects tens of thousands of anti-obesity compounds that have been withdrawn due to their serious side effects (biological activity vs. toxicity). Therefore, trends of recent studies have focused on screening natural products to explore functional core component resulting in body weight loss and reduction of levels of fat that generally have minimal adverse effects [
18]. In similar context, we have investigated the underlying molecular mechanism of
Artemisia annua L. (AWL), a potential anti-obesity effect, which could be associated with changes in a molecular level including transcriptional factors and regulatory pathway in adipocyte differentiation and lipogenesis using 3T3-L1 adipocytes as cellular models and HFD-induced obese rats in animal models.
Continuous efforts to elucidate molecular network of obesity accumulated evidence that a key feature of secondary metabolites from
Artemisia annua L. (AWL) possesses a number of biological activities [
19,
20]. For example, it can exhibit anti-proliferative effects or induce apoptosis in various tumour cell lines in vitro and in vivo [
21,
22]. Additionally,
Artemisia annua L. (AWL) has demonstrated significant anti-oxidant, anti-inflammatory, and anti-microbial properties [
12]. Recent studies also reported that artemisinic acid isolated from
Artemisia annua L. (AWL) inhibited adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells [
16]. Although numerous studies have demonstrated that AWL has a potential role as an anti-cancer therapy, there is limited information available about the anti-obesity effects of AWL.
As a key biological target, adipose tissue plays an important role as a storage depot for excess energy and regulates body energy homeostasis. Adipocyte hyperplasia and hypertrophy, as a result of inadequate adipogenesis in fat tissues, lead to obesity. Adipocyte differentiation from preadipocyte into mature adipocytes is a highly controlled process that involves changes in gene expression and the enlargement of intracellular lipid droplets [
23]. In the present study, we confirm inhibition of lipid accumulation by measuring intracellular triglyceride level staining with Oil Red O in 3T3-L1 adipocytes. This could lead to a significant reduction in adipogenesis in a dose-dependent manner of AWL without cell cytotoxicity. Furthermore, our in vivo study showed marked interventions in which AWL strongly reduced body weight gain, adipose mass, and adipocyte hypertrophy. Therefore, our findings suggest that AWL exerted anti-obesity effects in adipocytes and in HFD-induced obese rats.
Several transcription factors regulate adipocyte differentiation, in particular, C/EBPs and PPARγ, which synergistically activate the expression of adipocyte-specific genes to transform preadipocyte into mature adipocytes [
24]. PPARγ is a ligand-activated transcription factor that regulates adipogenesis during the early to terminal phase of differentiation. C/EBPα is expressed in the mid to late stages of adipogenesis and stimulates the differentiation of preadipocytes in cooperation with PPARγ [
6]. Moreover, PPARγ-deficient cells fail to differentiate into adipocytes, and overexpression of PPARγ, while C/EBPα accelerates adipogenesis [
25]. In this study, our findings unfold the molecular regulation of obesity by which AWL effectively inhibited the expression of C/EBPβ, C/EBPα, and PPARγ at the mRNA and protein levels. PPARγ and C/EBPα synergistically trans activate downstream adipocyte-specific gene expression, including aP2 enhancer, which is directly associated with lipogenic pathways [
26]. These results manifested the molecular link of obesity through the treatment of AWL inhibited the expression of aP2, which facilitate AWL driven suppression of adipogenesis via the downregulation of C/EBPα and PPARγ on molecular dysregulation in disease complications such as obesity.
Obesity causes insulin resistance, which may be associated with health complications such as diabetes, hyperlipidemia, and hypertension. There is a clear premise that the insulin signalling pathway plays a critical role in insulin-induced adipogenesis. Akt is an important signal mediator in the insulin-like growth factor 1 receptor signal cascade, which is involved in the induction of adipocyte differentiation [
27]. Ectopic expression of activated Akt induces the differentiation of 3T3-L1 pre-adipocytes into adipocytes [
28,
29]. Moreover, the overexpression of PPARγ in Akt-deficient mouse embryonic fibroblasts rescued their severe adipogenesis defect [
30], which supports the essential role of PPARγ induction downstream of Akt. In the present study, we found that AWL treatment induced a dose-dependent decrease in Akt phosphorylation and subsequently reduced the phosphorylation level of GSK3β. Both observations could be partly involved in the dysfunction of adipogenesis or lipogenesis. Emerging results including us, AWL-induced functional inactivate, such as inhibition of PPARγ and C/EBPα expression, might be influenced to the decreased level of Akt phosphorylation. Therefore, our results provide the first evidence that AWL inhibited insulin-mediated Akt phosphorylation, which, in turn, dramatically reduced adipogenic triglyceride accumulation by downregulating the PI3K/Akt pathway during the differentiation of 3T3-L1 preadipocytes into adipocytes.
Consistent with the inhibitory effect of AWL on adipocyte lipid accumulation, an in vivo study indicated that AWL prevented obesity in HFD-induced obese rats. The body weight of rats fed an HFD was lowered by 17% following administration of AWL compared to the HFD alone group. The reduced weight gain in AWL-fed rats was accompanied by a reduction in the weights of epididymal and perirenal adipose tissues. We examined the effect of AWL on adipogenesis- and/or obesity-related gene expression in adipose tissue of HFD-induced obese rats. The expressions of PPARγ and C/EBPα in adipose tissue of the AWL group were downregulated; subsequently, the gene expression of aP2 and FSA were decreased by treatment with AWL. Moreover, ACC, a PPAR-regulated fatty acid oxidation gene, was upregulated. Thus, our findings suggest that AWL significantly suppressed the expression of PPARγ and C/EBPα genes, which may regulate adipogenesis-related gene expression, fatty acid synthase, and lipogenesis in adipocytes. We also observed that AWL administration resulted in the improvement of numerous serum metabolic parameters, decreasing serum TG, TC, and increasing HDL-C levels, which are used as an indicator of adipocyte lipolysis. In addition, histological examination showed smaller fat cells in the epididymal fat tissue of the AWL-fed group in comparison to that in the HFD alone group, indicating that the decreased body weight gain was due to the reduced accumulation of fat. According to our observations, both natural and synthetic compounds from AWL shed light on new avenues of anti-obesity progression and/or blunt underlying obesity linked insulin resistance underlying metabolic health complications.