Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Treatments
2.2. Insulin Sensitivity Analysis
2.3. Western Blot Analysis
2.4. Transmission Electron Microscopy
2.5. Quantitative Real-Time PCR Analysis
2.6. Statistical Analysis
2.7. Serum Preparation and Metabolite Profiling
2.8. Metabolomics Data Processing and Multivariate Analysis
3. Results
3.1. Body Weight and Calorie Intake
3.2. Insulin Sensitivity
3.3. Serum Amino Acid Profiles
3.4. Serum Metabolic Profiles
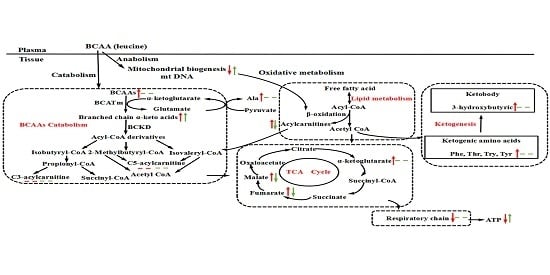
3.5. BCAA Catabolizing Enzymes and Metabolites
3.6. Mitochondrial Properties
4. Discussion and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Eller, L.K.; Saha, D.C.; Shearer, J.; Reimer, R.A. Dietary leucine improves whole-body insulin sensitivity independent of body fat in diet-induced obese Sprague-Dawley rats. J. Nutr. Biochem. 2013, 24, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nakamura, K.; Wada, K.; Tsuji, M.; Tamai, Y.; Kawachi, T. Branched-chain amino acid intake and the risk of diabetes in a japanese community: The takayama study. Am. J. Epidemiol. 2013, 178, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; D’Antona, G.; Nisoli, E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging (Albany NY) 2011, 3, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Nairizi, A.; She, P.; Vary, T.C.; Lynch, C.J. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J. Nutr. 2009, 139, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013, 62, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrere, B.; et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Liu, R.; Ma, Y.; Guo, H.; Hao, L.; Yao, P.; Liu, L.; Sun, X.; He, K.; et al. Chronic leucine supplementation increases body weight and insulin sensitivity in rats on high-fat diet likely by promoting insulin signaling in insulin-target tissues. Mol. Nutr. Food Res. 2013, 57, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Houde, V.P.; Brule, S.; Festuccia, W.T.; Blanchard, P.G.; Bellmann, K.; Deshaies, Y.; Marette, A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 2010, 59, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating bcaa levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; van Erk, M.; Verschuren, L.; van den Hoek, A.M.; Koek, M.; Wielinga, P.Y.; Jie, A.; Pellis, L.; Bobeldijk-Pastorova, I.; Kelder, T.; et al. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS ONE 2010, 5, e8817. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Macotela, Y.; Emanuelli, B.; Bang, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine—An environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermudez-Silva, F.J.; Elie, M.; Leste-Lasserre, T.; Belluomo, I.; Clark, S.; Duchampt, A.; Mithieux, G.; Cota, D. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity (Silver Spring) 2014, 22, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Yu, Y.H.; Hou, J.; Zhang, Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr. Metab. (Lond.) 2010, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Vaughan, R.A. Leucineinduced anabolic-catabolism: Two sides of the same coin. Amino Acids 2016, 48, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; McLean, L.L.; Philip, S.S.; Feldman, E.L. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology 2011, 152, 3638–3647. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Sullivan, K.A.; Backus, C.; Feldman, E.L. Cortical neurons develop insulin resistance and blunted akt signaling: A potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid. Redox Signal. 2011, 14, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; Tan, M.L.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol.Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Joshi, M.; Jeoung, N.H. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem. Biophys. Res. Commun. 2004, 313, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Obayashi, M.; Murakami, T.; Harris, R.A. Regulation of branched-chain amino acid catabolism: Nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Nellis, M.M.; Doering, C.B.; Kasinski, A.; Danner, D.J. Insulin increases branched-chain alpha-ketoacid dehydrogenase kinase expression in clone 9 rat cells. Am. J. Physiol.Endocrinol. Metab. 2002, 283, E853–E860. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Branched-chain amino acids: Enzyme and substrate regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [PubMed]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Hutson, S.M.; Sweatt, A.J.; Lanoue, K.F. Branched-chain [corrected] amino acid metabolism: Implications for establishing safe intakes. J. Nutr. 2005, 135, 1557S–1564S. [Google Scholar] [PubMed]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.M.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [PubMed]
- Affourtit, C. Mitochondrial involvement in skeletal muscle insulin resistance: A case of imbalanced bioenergetics. Biochim. Biophys. Acta 2016, 1857, 1678–1693. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Gannon, N.P.; Bisoffi, M.; Trujillo, K.A.; Conn, C.A. Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids 2013, 45, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zemel, M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. (Lond.) 2009, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Mermier, C.M.; Bisoffi, M.; Trujillo, K.A.; Conn, C.A. Dietary stimulators of the PGC-1 superfamily and mitochondrial biosynthesis in skeletal muscle. A mini-review. J. Physiol. Biochem. 2014, 70, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases sirt1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol.Endocrinol. Metab. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Mitochondria—A nexus for aging, calorie restriction, and sirtuins? Cell 2008, 132, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of enos. Science 2005, 310, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Roth, G.S. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp. Gerontol. 2011, 46, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Hong, T.; Wen, G.B.; Han, J.; Zuo, D.; Liu, Z.; Cao, W. Increased basal level of akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am. J. Physiol.Endocrinol. Metab. 2009, 297, E898–E906. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ning, J.; Yang, X.; Liu, Z. Excess exposure to insulin is the primary cause of insulin resistance and its associated atherosclerosis. Curr. Mol. Pharmacol. 2011, 4, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin resistance as a physiological defense against metabolic stress: Implications for the management of subsets of type 2 diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Fioravanti, M.; Locatelli, E.; Bonacasa, R.; Zamboni, M.; Basso, C.; Mazzoleni, A.; Mansi, V.; Geroutis, N.; Gazzaruso, C. Improvement of blood glucose control and insulin sensitivity during a long-term (60 weeks) randomized study with amino acid dietary supplements in elderly subjects with type 2 diabetes mellitus. Am. J. Cardiol. 2008, 101, 82E–88E. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; van Loon, L.J. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr. Rev. 2011, 69, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Nagao, Y.; Matsuoka, H.; Ide, T.; Sata, M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int. J. Mol. Med. 2008, 22, 105–112. [Google Scholar] [CrossRef] [PubMed]






| ND | HFD | HFD + Leu | |
|---|---|---|---|
| Macronutrients (g) | |||
| Protein | 20.3 | 20.3 | 20.3 |
| Carbohydrate | 64.5 | 44.6 | 44.6 |
| Fat | 4.5 | 24.4 | 24.4 |
| Fiber | 5.0 | 5.0 | 5.0 |
| Minerals | 3.0 | 3.0 | 3.0 |
| Calories (kcal) | 379.7 | 479.2 | 479.2 |
| Fat (energy %) | 10.7 | 45.8 | 45.8 |
| Protein (energy %) | 21.3 | 17.0 | 17.0 |
| Carbohydrate (energy %) | 68.0 | 37.2 | 37.2 |
| Amino acids (g) | |||
| Leucine | 1.3 | 1.5 | 2.9 |
| Isoleucine | 0.6 | 0.7 | 1.0 |
| Valine | 0.7 | 0.9 | 1.1 |
| Alanine | 0.8 | 0.8 | 0.5 |
| Aspartic acid | 1.5 | 1.6 | 1.2 |
| Glycine | 0.7 | 0.5 | 0.3 |
| Threonine | 0.7 | 0.7 | 0.7 |
| Serine | 0.8 | 1.0 | 0.8 |
| Glutamic acid | 3.4 | 3.2 | 3.2 |
| Tyrosine | 0.5 | 0.7 | 0.6 |
| Phenylalanine | 0.7 | 0.8 | 0.8 |
| Lysine | 1.1 | 1.2 | 1.2 |
| Histidine | 0.5 | 0.5 | 0.4 |
| Proline | 1.2 | 1.7 | 1.6 |
| Arginine | 1.0 | 0.5 | 0.6 |
| Methionine | 0.4 | 0.3 | 0.4 |
| Serine | 0.8 | 1.0 | 0.8 |
| Parameter | 24 weeks | 32 weeks |
|---|---|---|
| BCAAs | ||
| isoleucine | 1.01 (0.93, 1.09) | 0.97 (0.89, 1.06) |
| valine | 0.62 (0.55, 0.70) * | 1.04 (0.96, 1,11) |
| leucine | 0.70 (0.56, 0.84) * | 0.92 (0.84, 1.01) |
| Glucogenogenic amino acids | ||
| alanine | 0.48 (0.37, 0.59) * | 1.02 (0.89, 1.16) |
| asparagine | 0.72 (0.65, 0.80) * | 1.36 (1.14, 1.59) * |
| aspartic acid | 1.01 (0.60, 1.43) | 1.01 (0.83, 1.20) |
| cysteine | 1.06 (0.92, 1.20) | 1.33 (1.24, 1.42) * |
| glutamate | 0.79 (0.69, 0.90) * | 1.65 (1.38, 1.92) * |
| glutamine | 1.04 (0.98, 1.10) | 1.08 (1.02, 1.14) |
| glycine | 1.46 (1.24, 1.68) | 0.94 (0.83, 1.05) |
| histidine | 1.02 (0.95, 1.09) | 0.83 (0.77, 0.88) * |
| methionine | 1.01 (0.93, 1.08) | 1.09 (0.94, 1.24) |
| proline | 0.91 (0.84, 0.99) | 1.23 (1.06, 1.41) * |
| serine | 1.23 (1.04, 1.42) | 1.08 (1.02, 1.15) |
| arginine | 1.19 (1.06, 1.32) | 0.95 (0.83, 1.08) |
| Gluconeogenic and ketogenic amino acids | ||
| phenylalanine | 0.54 (0.48, 0.59) * | 1.10 (0.94, 1.25) |
| threonine | 1.05 (0.95, 1.15) | 1.34 (1.25, 1.42) * |
| tryptophan | 0.76 (0.68, 0.85) * | 0.90 (0.83, 0.98) |
| tyrosine | 1.12 (0.95, 1.29) | 0.89 (0.82, 0.96) |
| Ketogenic amino acids | ||
| lysine | 1.37 (1.20, 1.54) * | 1.46 (1.21, 1.71) * |
| 24 weeks | 32 weeks | |||
|---|---|---|---|---|
| Parameter | CR | HFD + Leu | CR | HFD + Leu |
| BCAAs | ||||
| isoleucine | 0.82 (0.76, 0.88) * | 0.91 (0.84, 0.98) | 0.88 (0.79, 0.96) | 0.94 (0.77, 1.11) |
| valine | 0.91 (0.86, 0.96) | 1.38 (1.18, 1.58) * | 0.94 (0.85, 1.03) | 0.95 (0.74, 1.15) |
| leucine | 0.85 (0.79, 0.91) * | 1.43 (1.02, 1.84) * | 0.91 (0.84, 0.98) | 0.98 (0.69, 1.27) |
| Glucogenogenic amino acids | ||||
| alanine | 0.79 (0.71, 0.87) * | 1.49 (1.34, 1.65) * | 1.29 (1.02, 1.56) * | 0.92 (0.71, 1.12) |
| asparagine | 0.91 (0.76, 1.06) | 1.49 (1.25, 1.72) * | 0.90 (0.73, 1.06) | 0.79 (0.60, 0.98) * |
| aspartic acid | 0.93 (0.82, 1.03) | 0.91 (0.74, 1.08) | 1.08 (0.96, 1.20) | 1.14 (0.97, 1.31) |
| cysteine | 1.19 (1.08, 1.30) * | 0.96 (0.85, 1.08) | 1.09 (0.93, 1.25) | 0.93 (0.77, 1.08) |
| glutamate | 0.93 (0.80, 1.07) | 1.07 (0.92, 1.23) | 1.08 (0.86, 1.31) | 1.00 (0.83, 1.17) |
| glutamine | 0.93 (0.85, 1.01) | 0.92 (0.84, 1.00) | 1.03 (0.88, 1.18) | 1.06 (0.91, 1.21) |
| glycine | 0.96 (0.90, 1.03) | 0.95 (0.91, 0.99) | 0.89 (0.81, 0.98) | 0.82 (0.71, 0.93) * |
| histidine | 0.98 (0.90, 1.05) | 0.97 (0.83, 1.11) | 1.09 (0.98, 1.20) | 1.05 (0.92, 1.18) |
| methionine | 0.94 (0.86, 1.03) | 1.01 (0.94, 1.08) | 0.94 (0.86, 1.02) | 0.97 (0.89, 1.04) |
| proline | 1.04 (0.80, 1.29) | 1.28 (1.00, 1.57) * | 1.24 (1.00, 1.49) * | 1.27 (1.02, 1.52) * |
| serine | 0.98 (0.91, 1.04) | 0.92 (0.85, 0.98) | 1.08 (0.98, 1.18) | 0.95 (0.84, 1.05) |
| arginine | 0.86 (0.82, 0.91) | 0.98 (0.84, 1.12) | 1.08 (1.00, 1.15) | 1.16 (0.96, 1.36) |
| Gluconeogenic and ketogenic amino acids | ||||
| phenylalanine | 0.89 (0.83, 0.95) * | 1.35 (1.20, 1.50) * | 0.94 (0.90, 0.97) | 1.07 (0.91, 1.21) |
| threonine | 1.17 (1.05, 1.29) | 0.92 (0.81, 1.02) | 1.08 (0.92, 1.25) | 1.09 (0.97, 1.21) |
| tryptophan | 1.00 (0.69, 1.31) | 1.43 (1.17, 1.69) * | 1.22 (0.94, 1.50) | 0.97 (0.77, 1.16) |
| tyrosine | 0.87 (0.71, 1.04) | 1.00 (0.87, 1.14) | 1.08 (0.92, 1.25) | 1.09 (0.97, 1.21) |
| Ketogenic amino acids | ||||
| lysine | 0.82 (0.74, 0.90) * | 0.65 (0.55, 0.74) * | 0.90 (0.75, 1.06) | 0.99(0.79, 1.19) |
| Metabolites | 24 weeks | 32 weeks |
|---|---|---|
| Amino acids derivatives | ||
| L-Kynurenine | 0.72 (0.64, 0.79) * | 1.09 (0.87, 1.31) |
| 5-hydroxytryptophan | 0.55 (0.44, 0.66) * | 1.11 (0.93, 1.29) * |
| Spermidine | 0.52 (0.48, 0.57) * | 1.26 (1.10, 1.42) |
| 2-hydroxyisobutyrate | 0.86 (0.77, 0.95) | 2.23 (1.87, 2.59) * |
| 2-hydroxyglutarate | 0.55 (0.47, 0.62) * | 1.37 (1.20, 1.54) * |
| S-(adenosyl)-L-homocysteine | 0.49 (0.41, 0.58) * | 1.28 (0.96, 1.60) |
| 4,6-dihydroxyquinoline | 0.55 (0.49, 0.62) * | 1.20 (0.93, 1.46) |
| Creatinine | 0.43 (0.33, 0.54) * | 1.19 (0.98, 1.40) |
| Creatine | 0.41 (0.31, 0.52) * | 1.34 (1.04, 1.64) * |
| Uridine | 0.39 (0.25, 0.52) * | 1.36 (1.17, 1.54) * |
| Fatty acid metabolism | ||
| 3-hydroxybutyric | 0.77 (0.70, 0.85) * | 1.92 (1.56, 2.27) * |
| Palmitic acid | 3.92 (3.25, 4.59) * | 1.53 (0.93, 2.12) |
| Acetylcarnitine | 0.70 (0.64, 0.77) * | 1.02 (0.84, 1.19) |
| Palmitoyl-L-carnitine | 0.91 (0.75, 1.08) | 1.48 (1.11, 1.86) |
| Carnitine | 0.46 (0.38, 0.54) * | 1.04 (0.68, 1.41) |
| Phospholipids | ||
| Lysophosphatidylcholine (C18:1) | 0.50 (0.43, 0.57) * | 1.18 (0.99, 1.37) |
| Lysophosphatidylcholine (C16:1) | 0.38 (0.34, 0.41) * | 0.99 (0.82, 1.16) |
| Lysophosphatidylcholine (C16:0) | 0.65 (0.58, 0.72) * | 0.97 (0.83, 1.12) |
| Lysophosphatidylcholine (C20:4) | 0.16 (0.12, 0.20) * | 1.21 (0.94, 1.48) |
| TCA cycle intermediates | ||
| Fumaric acid | 0.82 (0.74, 0.89) * | 1.12 (0.93, 1.31) |
| Malic acid | 3.54 (2.81, 4.27) * | 1.23 (1.07, 1.39) * |
| Citric acid | 0.91 (0.80, 1.03) | 1.22 (1.10, 1.34) * |
| a-Ketoglutaric acid | 0.52 (0.45, 0.59) * | 1.13 (0.98, 1.29) |
| Bile acid metabolism | ||
| Ursodeoxycholic acid | 0.28 (0.19, 0.38) * | 1.15 (0.73, 1.56) |
| Glycochenodeoxycholic acid | 0.32 (0.28, 0.35) * | 0.73 (0.60, 0.86) |
| Glycocholic acid | 0.71 (0.13, 1.28) | 0.65 (0.22, 1.08) |
| Purine metabolism | ||
| Xanthosine | 0.71 (0.46, 0.96) | 0.93 (0.71, 1.16) |
| Allantoin | 0.42 (0.33, 0.50) * | 1.00 (0.85, 1.14) |
| Uric acid | 0.88 (0.77, 0.98) | 1.10 (0.95, 1.25) |
| Xanthine | 0.83 (0.56, 1.09) | 1.00 (0.71, 1.29) |
| 24 weeks | 32 weeks | |||
|---|---|---|---|---|
| Metabolites | HFD + CR | HFD + Leu | HFD + CR | HFD + Leu |
| Amino acids derivatives | ||||
| L-Kynurenine | 1.30 (0.66, 1.95) | 1.70 (1.23, 2.19) * | 1.10 (0.43, 1.78) | 1.10 (0.81, 1.39) |
| 5-hydroxytryptophan | 1.10 (0.96, 1.25) | 1.58 (1.16, 1.92) * | 1.29 (0.82, 1.76) | 1.43 (1.07, 1.79) * |
| Spermidine | 1.17 (1.02, 1.33) * | 1.43 (1.26, 1.60) * | 1.18 (1.04, 1.32) * | 1.05 (0.89, 1.21) |
| 2-hydroxyisobutyrate | 0.90 (0.70, 1.10) | 1.54 (1.26, 1.81) * | 0.82 (0.56, 1.09) | 0.67 (0.38, 0.95) * |
| 2-hydroxyglutarate | 0.88 (0.73, 1.03) | 1.71 (1.50, 1.92) * | 1.07 (0.86, 1.28) | 1.01 (0.86, 1.16) |
| S-(adenosyl)-L-homocysteine | 1.24 (0.87, 1.60) | 1.57 (1.28, 1.86) * | 1.51 (1.20, 1.83) * | 1.34 (1.03, 1.66) * |
| 4,6-dihydroxyquinoline | 1.54 (1.21, 1.87) * | 1.81 (1.56, 2.06) * | 1.50 (1.17, 1.82) * | 0.90(0.75, 1.05) |
| Creatinine | 1.31 (1.19, 1.43) * | 1.50 (1.36, 1.64) * | 1.14 (0.91, 1.37) | 1.16 (0.91, 1.40) |
| Creatine | 0.91 (0.76, 1.06) | 1.33 (0.99, 1.68) | 1.04 (0.70, 1.37) | 1.71 (1.34, 2.08) * |
| Uridine | 1.32 (0.93, 1.72) | 1.61 (1.37, 1.85* | 1.02 (0.88, 1.17) | 0.92 (0.68, 1.17) |
| Fatty acid metabolism | ||||
| 3-hydroxybutyric | 0.92 (0.71, 1.13) | 1.36 (1.13, 1.59) * | 0.92 (0.60, 1.24) | 0.91 (0.48, 1.33) |
| Palmitic acid | 1.28 (1.03, 1.53) * | 1.81 (1.47, 2.15) * | 1.55 (1.17, 1.94) * | 0.54 (0.38, 0.70) * |
| Acetylcarnitine | 1.12 (0.96, 1.30) | 1.34 (1.21, 1.48) * | 1.15 (0.92, 1.39) | 0.87 (0.67, 1.07) |
| Palmitoyl-L-carnitine | 1.17 (0.97, 1.37) | 1.90 (1.54, 2.26) * | 0.99 (0.59, 1.40) | 0.49 (0.39, 0.58) * |
| Carnitine | 1.29 (1.09, 1.48) * | 1.86 (1.46, 2.26) * | 1.28 (1.09, 1.47) | 1.04 (0.93, 1.15) |
| Phospholipids | ||||
| Lysophosphatidylcholine (C18:1) | 1.35 (1.13, 1.56) * | 1.42 (1.19, 1.65) * | 1.55 (1.19, 1.90) * | 1.49 (1.14, 1.83) * |
| Lysophosphatidylcholine (C16:1) | 1.40 (1.15, 1.64) * | 1.42 (1.23, 1.60) * | 1.41 (1.00, 1.83) * | 0.91 (0.71, 1.10) |
| Lysophosphatidylcholine(C16:0) | 1.20 (1.05, 1.36) * | 1.34 (1.18, 1.49) * | 1.31 (1.13, 1.48) * | 1.21 (1.03, 1.40) * |
| Lysophosphatidylcholine (C20:4) | 1.06 (0.87, 1.25) | 1.04 (0.77, 1.30) | 0.87 (0.50, 1.24) | 3.82 (3.23, 4.41) * |
| TCA cycle intermediates | ||||
| Fumaric acid | 1.09 (0.93, 1.24) | 1.23 (1.08, 1.38) * | 1.09 (0.96, 1.22) | 0.82 (0.72, 0.93) * |
| Malic acid | 0.87 (0.74, 1.00) | 1.33 (1.11, 1.55) * | 0.97 (0.48, 1.47) | 0.15 (0.09, 0.22) * |
| Citric acid | 1.02 (0.90, 1.15) | 1.18 (0.97, 1.39) | 0.94 (0.77, 1.11) | 0.86 (0.74, 0.97) * |
| a-Ketoglutaric acid | 0.95 (0.80, 1.09) | 1.32 (1.13, 1.50) * | 1.23 (1.00, 1.47) | 1.05 (0.85, 1.25) |
| Bile acid metabolism | ||||
| Ursodeoxycholic acid | 1.57 (0.96, 2.19) | 2.58 (1.91, 3.24) * | 1.37 (1.06, 1.68) * | 1.35 (1.08, 1.61) * |
| Glycochenodeoxycholic acid | 1.45 (1.19, 1.71) * | 1.26 (0.92, 1.60) | 1.63 (1.13, 2.13) * | 1.43 (1.19, 1.66) * |
| Glycocholic acid | 2.20 (1.04, 3.36) * | 4.17 (0.60, 7.74) | 3.17 (1.08, 5.27) * | 3.76 (1.45, 6.07) * |
| Purine metabolism | ||||
| Xanthosine | 0.90 (0.63, 1.18) | 1.20 (0.77, 1.62) | 1.49 (1.13, 1.85) * | 1.05 (0.90, 1.19) |
| Allantoin | 1.05 (0.86, 1.24) | 1.33 (1.00, 1.66) * | 1.27 (1.04, 1.50) * | 1.41 (1.23, 1.59) * |
| Uric acid | 0.91 (0.77, 1.05) | 1.24 (1.07, 1.41) * | 1.08 (0.93, 1.23) | 0.90 (0.83, 0.96) |
| Xanthine | 1.05 (0.55, 1.55) | 1.26 (0.76, 1.76) | 1.71(1.38, 2.02) * | 1.12 (0.96, 1.27) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Li, H.; Fan, W.; Jin, Q.; Chao, T.; Wu, Y.; Huang, J.; Hao, L.; Yang, X. Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet. Nutrients 2017, 9, 565. https://doi.org/10.3390/nu9060565
Liu R, Li H, Fan W, Jin Q, Chao T, Wu Y, Huang J, Hao L, Yang X. Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet. Nutrients. 2017; 9(6):565. https://doi.org/10.3390/nu9060565
Chicago/Turabian StyleLiu, Rui, Hui Li, Wenjuan Fan, Qiu Jin, Tingting Chao, Yuanjue Wu, Junmei Huang, Liping Hao, and Xuefeng Yang. 2017. "Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet" Nutrients 9, no. 6: 565. https://doi.org/10.3390/nu9060565
APA StyleLiu, R., Li, H., Fan, W., Jin, Q., Chao, T., Wu, Y., Huang, J., Hao, L., & Yang, X. (2017). Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet. Nutrients, 9(6), 565. https://doi.org/10.3390/nu9060565