High Incidence and Levels of Ochratoxin A in Wines Sourced from the United States
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analytical Performance of HPLC-FD Coupled to LLE
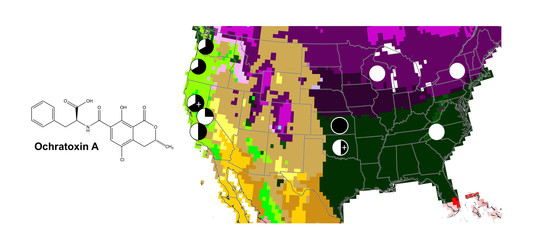
2.2. Incidence and Measured Levels of OTA in U.S. Wines
3. Conclusions
4. Materials and Methods
4.1. Wine Samples and Chemicals
4.2. Extraction and Sample Preparation for HPLC Analysis
4.3. HPLC-FD of OTA in Wines
4.4. Analytical Performance of HPLC-FD
4.5. HPLC-MS/MS of OTA in Wines
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Egmond, H.P.; Speijers, G.J.A. Survey of data on the incidence and levels of ochratoxin A in food and animal feed worldwide. J. Nat. Toxins 1994, 3, 125–143. [Google Scholar]
- Bennet, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E.H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Gonzalez, N.; Mintz, K.; Jaja-Chimedza, A.; De Jesus, C.L.; Lydon, C.; Welch, A.Z.; Berry, J.P. Teratogenicity of ochratoxin A, and the degradation product, ochratoxin α, in the zebrafish (Danio rerio) embryo model of vertebrate development. Toxins 2016, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Lino, C.M.; Pena, A. Mycotoxin food and feed regulation and the specific case of ochratoxin A: A review of the worldwide status. Food Addit. Contam. Part A 2010, 27, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- International Wine and Spirit Record (IWSR). Global Wine Consumption Set to Increase by 2 Billion Bottles. 2012. Available online: http://www.westernfarmpress.com/grapes/global-wine-consumption-set-increase-2-billion-bottles (accessed on 18 December 2017).
- McMillan, R. State of the Wine Industry. Silicon Valley Bank Wine Division. 2017. Available online: https://www.svb.com/uploadedFiles/Content/Trends_and_Insights/Reports/Wine_Report/2017-wine-report.pdf (accessed on 18 December 2017).
- Wine Institute. Available online: https://www.wineinstitute.org/resources/statistics/article86 (accessed on 18 December 2017).
- Abrunhosa, L.; Paterson, R.R.M.; Venancio, A. Biodegradation of ochratoxin A for food and feed decontamination. Toxins 2010, 2, 1078–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esti, M.; Benucci, I.; Liburdi, K.; Acciaro, G. Monitoring of ochratoxin A fate during alcoholic fermentation of wine-must. Food Control 2012, 2, 53–56. [Google Scholar] [CrossRef]
- Filali, A.; Ouammi, L.; Betbeder, A.M.; Baudrimont, I.; Soulaymani, R.; Benayada, A.; Creppy, E.E. Ochratoxin A in beverages from Morocco: A preliminary survey. Food Addit. Contam. 2001, 18, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Pietri, A.; Bertuzzi, T.; Pallaroni, L.; Piva, G. Occurrence of ochratoxin A in Italian wines. Food Addit. Contam. 2001, 18, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Assay of ochratoxin A in wine and beer by high-pressure liquid chromatography photodiode array and gas chromatography mass selective detection. J. Agric. Food Chem. 2001, 49, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Fabiani, A.; Sotckenström, S.; Mschicileli, N.; Sewram, V. Quantitation of ochratoxin A in South African wines. J. Agric. Food Chem. 2003, 51, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Siantar, D.; Halverson, C.A.; Kirmiz, C.; Peterson, G.F.; Hill, N.R.; Dugar, S.M. Ochratoxin A in wine: Survey by antibody- and polymeric-based SPE columns using HPLC/fluorescence detection. Am. J. Enol. Vitic. 2003, 54, 170–177. [Google Scholar]
- Soufleros, E.H.; Tricard, C.; Bouloumpasi, E. Occurrence of ochratoxin A in Greek wines. J. Sci. Food Agric. 2003, 83, 173–179. [Google Scholar] [CrossRef]
- Stefanaki, I.; Foufa, E.; Tsatsou-Dritsa, A.; Dais, P. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit. Contam. 2003, 20, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.; Mankotia, M.; Pantazopoulos, P.; Neil, R.J.; Scott, P.M. Ochratoxin A in wine and grape juice sold in Canada. Food Addit. Contam. 2004, 21, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.A.R.; Magnoli, C.E.; Fraga, M.E.; Dalcero, A.M.; Santana, D.M.N. Occurrence of ochratoxin A in wine and grape juice marketed in Rio de Janeiro, Brazil. Food Addit. Contam. 2004, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Anli, E.; Cabuk, B.; Vural, N.; Baspinar, E. Ochratoxin in Turkish wines. J. Food Biochem. 2005, 29, 611–623. [Google Scholar] [CrossRef]
- Belajova, E.; Rauova, D. Determination of ochratoxin A and its occurrence in wines of Slovakian retail. J. Food Nutr. Res. 2007, 46, 68–74. [Google Scholar]
- Burdaspal, P.; Legarda, T. Occurrence of ochratoxin A in sweet wine produced in Spain and other countries. Food Addit. Contam. 2007, 24, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Nicoletti, I.; Pascale, M.; De Rossi, A.; De Girolamo, A.; Visconti, A. Positive correlation between high levels of ochratoxin A and resveratrol-related compounds in red wines. J. Agric. Food Chem. 2007, 55, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Brera, C.; Debegnach, F.; Minardi, V.; Prantera, E.; Pannunzi, E.; Faleo, S.; de Santis, B.; Miraglia, M. Ochratoxin A contamination in Italian wine samples and evaluation of the exposure in the Italian population. J. Agric. Food Chem. 2008, 56, 10611–10618. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Marín, S.; Ramos, A.J.; Sanchis, V. Survey: Ochratoxin A in European special wines. Food Chem. 2008, 108, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Altiokka, G.; Can, N.O.; Atkosar, Z.; Aboul-Enein, H.Y. Determination of ochratoxin A in Turkish wines. J. Food Drug Anal. 2009, 17, 467–473. [Google Scholar]
- Flajs, D.; Domijan, A.M.; Ivic, D.; Cvjetkovic, B.; Peraica, M. ELISA and HPLC analysis of ochratoxin A in red wines of Croatia. Food Control 2009, 20, 590–592. [Google Scholar] [CrossRef]
- Radoi, A.; Dumitru, L.; Barthelmebs, L.; Marty, J.L. Ochratoxin A in some French wines: Application of a dirct competitive ELISA based on an OTA-HRP conjugate. Anal. Lett. 2009, 42, 1187–1202. [Google Scholar] [CrossRef]
- Pena, A.; Cerejo, F.; Silva, L.J.; Lino, C.M. Ochratoxin A survey in Portugese wine by LC-FD with direct injection. Talanta 2010, 82, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Ponsone, M.L.; Chiotta, M.L.; Combina, M.; Torres, A.; Knass, P.; Dalcero, A.; Chulze, S. Natural occurrence of ochratoxin A in musts, wines and grape vine fruits from grapes harvested in Argentina. Toxins 2010, 2, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Loré, A.; Garibaldi, A.; Gullino, M.L. Occurrence of ochratoxin A before bottling in DOC and DOCG wines produced in Piedmont (Northern Italy). Food Control 2010, 21, 1294–1297. [Google Scholar] [CrossRef]
- Labrinea, E.P.; Natskoulis, P.I.; Spiropoulos, A.E.; Magan, N.; Tassou, C.C. A survey of ochratoxin A occurrence in Greek wines. Food Addit. Contam. Part B Surveill. 2011, 4, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, Y.; Wang, Y.; Xu, R. Occurrence of ochratoxin A in wine and beer samples from China. Food Addit. Contam. Part B Surveill. 2011, 4, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Mikulikova, R.; Belakova, S.; Benesova, K.; Svoboda, Z. Study of ochratoxin A content in South Moravian wines by the UPLC method with fluorescence detection. Food Chem. 2012, 133, 55–59. [Google Scholar] [CrossRef]
- Vega, M.; Rios, G.; von Baer, D.; Mardones, C.; Tessini, C.; Herlitz, E.; Saelzer, R.; Ruiz, M.A. Ochratoxin A occurrence in wines produced in Chile. Food Control 2012, 28, 147–150. [Google Scholar] [CrossRef]
- Lasram, S.; Oueslati, S.; Chebil, S.; Mliki, A.; Ghorbel, A. Occurrence of ochratoxin A in domestic beer and wines from Tunisia by immunoaffinity clean-up and liquid chromatography. Food Addit. Contam. Part B Surveill. 2013, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mariño-Repizo, L.; Gargantini, R.; Manzano, H.; Raba, J.; Cerutti, S. Assessment of ochratoxin A occurrence in Argentine red wines using a novel sensitive QuEChERS-solid phase extraction approach prior to ultra high performance liquid chromatography-tandem mass spectrometry methodology. J. Sci. Food Agric. 2016, 97, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Skarkova, J.; Ostry, V.; Malir, F.; Roubal, T. Determination of ochratoxin A in food by high performance liquid chromatography. Anal. Lett. 2013, 46, 1495–1504. [Google Scholar] [CrossRef]
- Al-Taher, F.; Banaszewski, K.; Jackson, L.; Zweigenbaum, J.; Ryu, D.; Cappozzo, J. Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J. Agric. Food Chem. 2013, 61, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Z.; Hu, X.; Zhang, Q. Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects. Mass Spectrom. Rev. 2013, 32, 420–452. [Google Scholar] [CrossRef] [PubMed]
- Rizzazzi-Fazeli, E.; Reiter, E.V. Sample preparation and clean up strategies in the mycotoxin analysis: Principles, applications and recent developments. In Determining Mycotoxins and Mycotoxigenic Fungi in Food and Feed; De Saeger, S., Ed.; Wood Publishing: Cambridge, UK, 2011; pp. 37–70. [Google Scholar]
- Fabiani, A.; Corzani, C.; Arfelli, G. Correlation between different clean-up methods and analytical techniques performances to detect ochratoxin A in wine. Talanta 2010, 8, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Perez, J.F.; Arroyo-Manzanares, N.; Garcia-Campaña, A.M.; Gamiz-Gracia, L. Solid-phase extraction as sample treatment for the determination of ochratoxin A in foods: A review. Crit. Rev. Food Sci. Nutr. 2016, 57, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzares, N.; Garcia-Campaña, A.M.; Gamiz-Gracia, L. Comparison of different sample treatments for the analysis of ochratoxin A in wine by capillary HPLC with laser-induced fluorescence detection. Anal. Bioanal. Chem. 2011, 401, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Pizzutti, I.R.; de Kok, A.; Scholten, J.; Righi, L.W.; Cardoso, C.D.; Rohers, G.N.; da Silva, R.C. Development, optimization and validation of a multimethod for the determination of 36 mycotoxins in wines by liquid chromatography-tandem mass spectrometry. Talanta 2014, 129, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Pascale, M.; Centonze, G. Determination of ochratoxin A in wine by means of immunoaffinity column clean-up and high-performance liquid chromatography. J. Chromatogr. A 1999, 864, 89–101. [Google Scholar] [CrossRef]
- Tunçel, M.; Öncü Kaya, E.M.; Uysal, U.D.; Güray, T. HPLC fluorescence determination of ochratoxin A utilizing double internal standard and its application to poultry feed. Turk. J. Chem. 2015, 39, 372–381. [Google Scholar] [CrossRef]
- Leitner, A.; Zöllner, P.; Paolillo, A.; Stroka, J.; Papadopoulou-Bouraoui, A.; Jaborek, S. Comparison of methods for determination of ochratoxin A in wine. Anal. Chim. Acta 2002, 453, 33–41. [Google Scholar] [CrossRef]
- Roland, A.; Bros, P.; Bouisseau, A.; Cavelier, F.; Schneider, R. Analysis of ochratoxin A in grapes, musts and wines by LC-MS/MS: First comparison of stable isotope dilution assay and diastereomeric dilution assay methods. Anal. Chim. Acta 2014, 818, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Battiliani, P.; Magan, N.; Logrieco, A. European research on ochratoxin A in grapes and wine. Int. J. Food Microbiol. 2006, 111, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.; Medina, A.; Mateo, E.M.; Mateo, F.; Jimenez, M. An overview of ochratoxin A in beer and wine. Int. J. Food Microbiol. 2007, 119, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Perrone, G.; Cozzi, G.; Solfrizzo, M. Managing ochratoxin A risk in the grape-wine food chain. Food Addit. Contam. A 2008, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Spellman, G. Wine, weather and climate. Weather 1999, 54, 230–239. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Zimmerli, B.; Dick, R. Ochratoxin A in table wine and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996, 13, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Ottender, H.; Majerus, P. Occurrence of ochratoxin A (OTA) in wines: Influences of the type of wine and its geographical origin. Food Addit. Contam. 2000, 17, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Remiro, R.; Irigoyen, A.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Levels of ochratoxins A in Mediterranean red wines. Food Control 2013, 32, 63–68. [Google Scholar] [CrossRef]
- Kos, J.; Mastilovic, J.; Hajinal, E.J.; Saric, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Control 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Dobolyi, C.; Sebok, F.; Varga, J.; Kocsube, S.; Szigeti, G.; Baranyi, N.; Szecsi, A.; Toth, B.; Varga, M.; Kriszt, B.; et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in Hungary. Acta Aliment. 2013, 42, 451–459. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Thermophilic fungi dominate aflatoxigenic/mycotoxigenic fungi on food under global warming. Int. J. Environ. Res. Public Health 2017, 14, 199. [Google Scholar] [CrossRef] [PubMed]


| Type (# Wines) | >LOD (%) | Mean | Median | Range | 1 Number (%) of Wines in the Concentration Range, µg L−1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <0.3 | 0.3–0.5 | 0.5–1.0 | >1.0 2 | >2.0 3 | >5.0 | |||||
| Red (31) | 28 (90.3) | 1.0 | 1.0 | 0.3–2.1 | 3 (9.7) | 2 (6.5) | 6 (19.4) | 17 (54.8) | 1 (3.2) | 0 (0.0) |
| Sweet (3) | 2 (66.7) | 0.8 | 0.7 | 0.5–1.1 | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) |
| Dry (28) | 26 (92.9) | 1.0 | 1.1 | 0.3–2.1 | 3 (10.7) | 2 (7.1) | 5 (17.9) | 16 (57.1) | 0 (0) | 0 (0.0) |
| White (10) | 7 (70.0) | 2.6 | 1.2 | 0.6–8.6 | 1 (10.0) | 0 (0.0) | 2 (20.0) | 4 (40.0) | 1 (10.0) | 1 (10.0) |
| Sweet (7) | 4 (57.1) | 5.0 | 5.0 | 1.4–8.6 | 1 (14.3) | 0 (0.0) | 1 (14.3) | 2 (28.6) | 1 (14.3) | 1 (14.3) |
| Dry (3) | 3 (100.0) | 0.9 | 1.1 | 0.6–1.2 | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (66.7) | 0 (0.0) | 0 (0.0) |
| Sweet (10) | 6 (60.0) | 2.5 | 1.1 | 0.5–8.6 | 1 (10.0) | 0 (0.0) | 2 (20.0) | 3 (30.0) | 1 (10.0) | 1 (0.0) |
| Dry (31) | 29 (93.5) | 1.0 | 1.1 | 0.3–2.1 | 3 (9.7) | 2 (6.5) | 6 (19.4) | 18 (58.1) | 1 (3.2) | 0 (0.0) |
| Total (41) | 35 (85.4) | 1.3 | 1.1 | 0.3–8.6 | 4 (9.8) | 2 (4.9) | 8 (19.5) | 21 (51.2) | 2 (4.9) | 1 (2.4) |
| Region | Range (µg L−1) | >2 µg L−1 # Wines (%) | Analytical Method (Sample Preparation) | Reference |
|---|---|---|---|---|
| North America | ||||
| U.S.A. | 0.3–8.6 | 2/41 (4.9%) | HPLC-FD (LLE) | Present study |
| 0.01–1.68 | - | HPLC-FD (IAC) | [16] | |
| Canada | 0.050–0.393 | - | HPLC-FD (IAC) | [19] |
| Europe | ||||
| Italy | 0.2–3.177 | N/A | HPLC-FD (IAC) | [13] |
| 0.1–7.63 | 6/55 (10.9%) | HPLC-FD (IAC) | [48] | |
| 0.2–4.93 | 9/112 (8.0%) | HPLC-FD (IAC) | [24] | |
| 0.03–7.50 | 22/783 (2.8%) | HPLC-FD (IAC) | [25] | |
| 0.01–2.63 | 29/1206 (2.4%) | HPLC-FD (IAC) | [32] | |
| Greece | 0.05–2.00 | 1/105 (<1.0%) | HPLC-FD (IAC) | [33] |
| 0.05–2.69 | N/A | HPLC-FD (IAC) | [18] | |
| 0.02–3.20 | 3/35 (8.6%) | HPLC-FD (IAC) | [17] | |
| Spain | 0.02–4.63 | 18/188 (9.6%) | HPLC-FD (IAC) | [23] |
| Croatia | 0.014–0.021 | - | HPLC-FD (IAC) | [28] |
| Turkey | 0.01–2.3 | 1/47 (2.1%) | HPLC-FD (LLE/SPE) | [21] |
| [27] | ||||
| Slovakia | 0.036–0.463 | - | HPLC-FD (IAC) | [22] |
| Moravia | 0.001–0.072 | - | HPLC-FD (IAC) | [35] |
| Portugal | 1.23–2.4 | 1/60 (1.7%) | HPLC-FD (direct inject) | [30] |
| France | 0.31–0.92 | - | HPLC-FD (IAC) | [29] |
| South America | HPLC-FD (IAC) | |||
| Argentina | 0.028–0.042 | - | HPLC-FD (IAC) | [20] |
| 2.00–4.82 | 3/47 (6.4%) | HPLC-FD (IAC) | [31] | |
| 0.05–0.98 | - | HPLC-MS/MS (SPE) | [38] | |
| Chile | 0.028–0.071 | - | HPLC-FD (IAC) | [20] |
| 0.14–0.35 | - | HPLC-FD (IAC) | [36] | |
| Brazil | 0.028–0.042 | - | HPLC-FD (IAC) | [20] |
| Africa | ||||
| South Africa | 0.04–0.39 | - | HPLC-FD (IAC) | [15] |
| Tunisia | 0.09–1.50 | - | HPLC-FD (IAC) | [37] |
| Morocco | 0.028–3.24 | 1/30 (3.3%) | HPLC-FD (SPE) | [12] |
| Asia | ||||
| China | 0.09–1.18 | - | HPLC-FD (IAC) | [34] |
| State (# Wines) 1 | Region (# Wines) | >LOQ (%) | Average [OTA], µg L−1 | Range 2, µg L−1 |
|---|---|---|---|---|
| CA (24) | 21 (87.5%) | 1.0 | 0.3–2.1 | |
| Central Coast (2) | 2 (100.0%) | 0.9 | 0.7–1.1 | |
| Central Valley (4) | 3 (75.0%) | 1.0 | 0.6–1.4 | |
| North Coast (18) | 16 (88.9%) | 1.0 | 0.3–2.1 | |
| KS (1) | 1 (100.0%) | 1.4 | ≤1.4 | |
| NC (1) | 1 (100.0%) | 1.0 | ≤1.0 | |
| NY (3) | 1 (33.3%) | 0.5 | ≤0.5 | |
| OK (2) | 1 (50%) | 8.6 | ≤8.6 | |
| OR (3) | 2 (66.7%) | 1.4 | 1.3–1.5 | |
| WA (3) | 3 (100%) | 0.9 | 0.6–1.1 | |
| WI (2) | 0 (0%) | <LOQ | N/A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesus, C.L.; Bartley, A.; Welch, A.Z.; Berry, J.P. High Incidence and Levels of Ochratoxin A in Wines Sourced from the United States. Toxins 2018, 10, 1. https://doi.org/10.3390/toxins10010001
De Jesus CL, Bartley A, Welch AZ, Berry JP. High Incidence and Levels of Ochratoxin A in Wines Sourced from the United States. Toxins. 2018; 10(1):1. https://doi.org/10.3390/toxins10010001
Chicago/Turabian StyleDe Jesus, Christopher Lawrence, Amanda Bartley, Aaron Z. Welch, and John P. Berry. 2018. "High Incidence and Levels of Ochratoxin A in Wines Sourced from the United States" Toxins 10, no. 1: 1. https://doi.org/10.3390/toxins10010001
APA StyleDe Jesus, C. L., Bartley, A., Welch, A. Z., & Berry, J. P. (2018). High Incidence and Levels of Ochratoxin A in Wines Sourced from the United States. Toxins, 10(1), 1. https://doi.org/10.3390/toxins10010001