Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Detection of Pacific Ciguatoxins in Toxic Samples from Nuku Hiva Island (French Polynesia)
Abstract
:1. Introduction
2. Results
2.1. Abundance of Gambierdiscus spp. in Study Sites
2.2. Toxicity Results Using CBA-N2a
2.2.1. Calibration of CBA-N2a
2.2.2. Toxicity of Gambierdiscus In Vitro Cultures
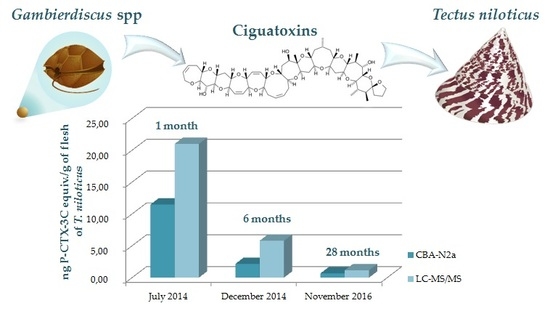
2.2.3. Toxicity of T. niloticus Samples
2.3. Toxin Profiles in Tectus niloticus Toxic Fractions Using LC-MS/MS
2.3.1. Detection of Pacific Ciguatoxins in T. niloticus from Nuku Hiva
2.3.2. Screening of T. niloticus Samples for Other Marine Toxins
3. Discussion
4. Materials and Methods
4.1. Study Sites
4.2. Biological Material and Sampling Procedures
4.2.1. Gambierdiscus Samples and qPCR Assays
4.2.2. Tectus niloticus Samples
4.3. Extraction Procedures
4.4. Cell-Based Assay Using Neuroblastoma Cells
4.5. Liquid Chromatography Coupled with Tandem Mass Spectrometry
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Williams, S.T.; Karube, S.; Ozawa, T. Molecular systematics of vetigastropoda: Trochidae, turbinidae and trochoidea redefined. Zool. Scr. 2008, 48, 1–24. [Google Scholar] [CrossRef]
- Purcell, S.W.; Cheng, Y.W. Experimental restocking and seasonal visibility of a coral reef gastropod assessed by temporal modelling. Aquat. Biol. 2010, 9, 227–238. [Google Scholar] [CrossRef]
- Lorrain, A.; Clavier, J.; Thébault, J.; Tremblay-Boyer, L.; Houlbrèque, F.; Amice, E.; Le Gof, M.; Chauvaud, L. Variability in diel and seasonal in situ metabolism of the tropical gastropod Tectus niloticus. Aquat. Biol. 2015, 23, 167–182. [Google Scholar] [CrossRef]
- Pakoa, K.; Friedman, K.; Damlamian, H. The status of trochus (Trochus niloticus) in Tongatapu Lagoon, kingdom of Tonga. SPC Trochus Inf. Bull. 2010, 15, 3–16. [Google Scholar]
- Castell, L.L. Population studies of juvenile Trochus niloticus on a reef flat on the North-Eastern Queensland coast, Australia. Mar. Freshw. Res. 1997, 48, 211–217. [Google Scholar] [CrossRef]
- Hoang, D.H.; Tuyen, H.T.; Lu, H.D. Growth rate of Trochus niloticus (l., 1767) fed different food types. SPC Trochus Inf. Bull. 2008, 14, 7–11. [Google Scholar]
- Villanueva, R.D.; Baria, M.V.B.; de la Cruz, D.W. Effects of grazing by herbivorous gastropod (Trochus niloticus) on the survivorship of cultured coral spat. Zool. Stud. 2013, 52. [Google Scholar] [CrossRef]
- Bour, W. Un Mollusque Nacrier du Pacifique. Biologie, Écologie et Gestion Rationnelle d’un Mollusque Nacrier du Pacifique: Le Troca (Trochus niloticus L.) de Nouvelle Calédonie; Editions de l’ORSTOM, Collection Etudes et Thèses; Institut Francais de Recherche Scientifique pour le Développement en Coopération: Paris, France, 1992; p. 174. [Google Scholar]
- Adams, T.J.H.; Dalzell, P.J. Artisanal Fishing. In East-West Center Workshop on Marine Biodiversity; University of Hawaii: Honolulu, HI, USA, 1994; p. 19. [Google Scholar]
- Hoang, D.H.; Tuan, V.S.; Hoa, N.X.; Sang, H.M.; Lu, H.D.; Tuyen, H.T. Experiments on using hatchery-reared Trochus niloticus juveniles for stock enhancement in Vietnam. SPC Trochus Inf. Bull. 2007, 13, 13–18. [Google Scholar]
- Teitelbaum, A.; Friedman, K. Successes and failures in reintroducing giant clams in the Indo-Pacific region. SPC Trochus Inf. Bull. 2008, 14, 19–26. [Google Scholar]
- Tuhumury, F.S. Dynamic model of Trochus niloticus. Linn, in resources management, in coastal area of Saparua Island, Saparua Subdistrict, Central Maluku Regency. IOSR J. Agric. Vet. Sci. 2014, 7, 33–40. [Google Scholar] [CrossRef]
- Gillett, R. Pacific islands trochus introductions 1927–1998. SPC Trochus Inf. Bull. 2002, 9, 9–13. [Google Scholar]
- French Polynesia. Délibération relative à la protection de certaines espèces animales marines et d’eau douce du patrimoine naturel polynésien. Journal Officiel de la Polynésie Française: Tahiti, French Polynesia, 1988.
- Gatti, C.; Lonatti, D.; Darius, H.T.; Chinain, M. First report of a mass-poisoning outbreak following the consumption of Tectus niloticus (Gastropod) in French Polynesia: A novel pathway of Ciguatera Shellfish Poisoning? Harmful Algae News 2015, 50, 19–20. [Google Scholar]
- Lonati, D.; Gatti, C.M.; Zancan, A.; Darius, H.T.; Fleure, M.; Chinain, M.; Buonocore, M.; Locatelli, C.A. Novel ciguatera shellfish poisoning (CSP) cluster following the consumption of Tectus niloticus (gastropod) in Nuku Hiva (Marquesas Archipelago, French Polynesia). In Proceedings of the 35th International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT), St Julian’s, Malta, 26–29 May 2015; Clinical Toxicology: St Julian’s, Malta, 2015; Volume 53, p. 278. [Google Scholar]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Laurent, D.; Kerbrat, A.S.; Darius, H.T.; Rossi, F.; Yeeting, B.; Haddad, M.; Golubic, S.; Pauillac, S.; Chinain, M. Ciguatera shellfish poisoning (CSP): A new ecotoxicological phenomenon from cyanobacteria to humans via giant clams. In Food Chains: New Research; Melissa, A.J., Danielle, W.M., Eds.; Nova Science Publishers, Inc.: NewYork, NY, USA, 2012; Chapter 1; pp. 1–43. [Google Scholar]
- Chinain, M.; Darius, H.T.; Ung, A.; Tchou Fouc, M.; Revel, T.; Cruchet, P.; Pauillac, S.; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2010, 56, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Pawlowiez, R.; Darius, H.T.; Cruchet, P.; Rossi, F.; Caillaud, A.; Laurent, D.; Chinain, M. Evaluation of seafood toxicity in the Australes Archipelago (French Polynesia) using the neuroblastoma cell-based assay. Food Addit. Contam. Part A 2013, 30, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Rongo, T.; van Woesik, R. Ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2011, 10, 345–355. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 1627, 1–38. [Google Scholar]
- Food and Drug Administration. Fish and Fishery Products Hazards and Control Guidance. Available online: https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/seafood/ucm2018426.htm (accessed on 13 December 2017).
- Rehmann, N.; Hess, P.; Quilliam, M.A. Discovery of new analogs of the marine biotoxin azaspiracid in blue mussels (Mytilus edulis) by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Shumway, S.E. Phycotoxin-related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci. 1995, 3, 1–31. [Google Scholar] [CrossRef]
- Negri, A.; Llewellyn, L. Comparative analyses by HPLC and the sodium channel and saxiphilin 3H-saxitoxin receptor assays for paralytic shellfish toxins in crustaceans and molluscs from tropical North West Australia. Toxicon 1998, 36, 283–298. [Google Scholar] [CrossRef]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Costa, S.T.; Braga, A.C.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine bio toxins in nonbivalve vectors. Food Control 2017, 76, 24–33. [Google Scholar] [CrossRef]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouc, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Roué, M.; Darius, H.T.; Viallon, J.; Ung, A.; Gatti, C.; Harwood, T.; Chinain, M. Application of solid phase adsorption toxin tracking (SPATT) devices for the field detection of Gambierdiscus toxins. Harmful Algae 2018, 71, 40–49. [Google Scholar] [CrossRef]
- Holland, W.C.; Litaker, W.; Tomas, C.; Kibler, S.; Place, A.; Davenport, E.; Tester, P. Differences in the toxicity of six Gambierdiscus (Dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon 2013, 65, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, T.D.; Smith, K.F.; Meyer, L.; Capper, A.; Brett, S.; Hallegraeff, G.M. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaiani, G.; Ferron, P.-J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, R.W.; et al. Maitotoxin-4, a novel MTX analog produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Germain, M.; Deparis, X.; Pauillac, S.; Legrand, A.-M. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp. (Dinophyceae), the causative agent of ciguatera in Tahiti, French Polynesia. Mar. Biol. 1999, 135, 259–267. [Google Scholar] [CrossRef]
- Dickey, R.W.; Plakas, S.M. Ciguatera: A public health perspective. Toxicon 2010, 56, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Kibler, S.R.; Litaker, R.W.; Holland, W.C.; Vandersea, M.W.; Tester, P.A. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae 2012, 19, 1–14. [Google Scholar] [CrossRef]
- Yoshimatsu, T.; Yamaguchi, H.; Iwamoto, H.; Nishimura, T.; Adachi, M. Effects of temperature, salinity and their interaction on growth of Japanese Gambierdiscus spp. (Dinophyceae). Harmful Algae 2014, 35, 29–37. [Google Scholar] [CrossRef]
- Xu, Y.; Richlen, M.L.; Liefer, J.D.; Robertson, A.; Kulis, D.; Smith, T.B.; Parsons, M.L.; Anderson, D.M. Influence of environmental variables on Gambierdiscus spp. (Dinophyceae) growth and distribution. PLoS ONE 2016, 11, e0153197. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, L.; Momigliano, P.; Russ, G.R.; Heimann, K. Effects of temperature, salinity and composition of the dinoflagellate assemblage on the growth of Gambierdiscus carpenteri isolated from the great barrier reef. Harmful Algae 2017, 65, 52–60. [Google Scholar] [CrossRef] [PubMed]
- John, U.; Tillmann, U.; Hülskötter, J.; Alpermann, T.J.; Wohlrab, S.; Van de Waal, D.B. Intraspecific facilitation by allelochemical mediated grazing protection within a toxigenic dinoflagellate population. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Portela, M.; Riobó, P.; Franco, J.M.; Bañuelos, R.M.; Rodríguez, F. Genetic and toxinological characterization of north atlantic strains of the dinoflagellate Ostreopsis and allelopathic interactions with toxic and non-toxic species from the genera Prorocentrum, Coolia and Gambierdiscus. Harmful Algae 2016, 60, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Towers, N.; Briggs, L.; Munday, R.; Adamson, J. Uptake of palytoxin-like compounds by shellfish fed Ostreopsis siamensis (Dinophyceae). N. Z. J. Mar. Freshw. Res. 2002, 36, 631–636. [Google Scholar] [CrossRef]
- Rolland, J.L.; Pelletier, K.; Masseret, E.; Rieuvilleneuve, F.; Savar, V.; Santini, A.; Amzil, Z.; Laabir, M. Paralytic toxins accumulation and tissue expression of a-amylase and lipase genes in the pacific oyster Crassostrea gigas fed with the neurotoxic dinoflagellate Alexandrium catenella. Mar. Drugs 2012, 10, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Jauffrais, T.; Marcaillou, C.; Herrenknecht, C.; Truquet, P.; Sechet, V.; Nicolau, E.; Tillmann, U.; Hess, P. Azaspiracid accumulation, detoxification and biotransformation in blue mussels (Mytilus edulis) experimentally fed Azadinium spinosum. Toxicon 2012, 60, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Jauffrais, T.; Kilcoyne, J.; Herrenknecht, C.; Truquet, P.; Sechet, V.; Miles, C.O.; Hess, P. Dissolved azaspiracids are absorbed and metabolized by blue mussels (Mytilus edulis). Toxicon 2013, 65, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, H.; Lambert, C.; Le Goic, N.; Quere, C.; Bruneau, A.; Riso, R.; Auffret, M.; Soudant, P. Cellular and biochemical responses of the oyster Crassostrea gigas to controlled exposures to metals and Alexandrium minutum. Aquat. Toxicol. 2014, 147, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Laurent, D.; Kerbrat, A.-S.; Darius, H.T.; Girard, E.; Golubic, S.; Benoit, E.; Sauviat, M.-P.; Chinain, M.; Molgo, J.; Pauillac, S. Are cyanobacteria involved in Ciguatera Fish Poisoning-like outbreaks in new Caledonia? Harmful Algae 2008, 7, 827–838. [Google Scholar] [CrossRef]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Rodriguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. First report of ciguatoxins in two starfish species : Ophidiaster ophidianus and Marthasterias glacialis. Toxins 2015, 7, 3740–3757. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.-S.; Darius, H.T.; Pauillac, S.; Chinain, M.; Laurent, D. Detection of ciguatoxin-like and paralysing toxins in Trichodesmium spp. from New Caledonia lagoon: New Caledonia tropical lagoons: An overview of multidisciplinary investigations. Mar. Pollut. Bull. 2010, 61, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Mejean, A.; Peyraud-Thomas, C.; Kerbrat, A.S.; Golubic, S.; Pauillac, S.; Chinain, M.; Laurent, D. First identification of the neurotoxin homoanatoxin-a from mats of Hydrocoleum lyngbyaceum (marine cyanobacterium) possibly linked to giant clam poisoning in New Caledonia. Toxicon 2010, 56, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.-S.; Amzil, Z.; Pawlowiez, R.; Golubic, S.; Sibat, M.; Darius, H.T.; Chinain, M.; Laurent, D. First evidence of palytoxin and 42-hydroxy-palytoxin in the marine cyanobacterium Trichodesmium. Mar. Drugs 2011, 9, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, A.; Laurent, D.; Chinain, M.; Gugger, M.; Humbert, J.F. Molecular characterization of the diversity and potential toxicity of cyanobacterial mats in two tropical lagoons in the South Pacific ocean. J. Phycol. 2012, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Vandersea, M.W.; Kibler, S.R.; Holland, W.C.; Tester, P.A. Development of semi-quantitative pcr assays for the detection and enumeration of Gambierdiscus species (Gonyaulacales, Dinophyceae). J. Phycol. 2012, 48, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Hendriksen, P.J.M.; Gerssen, A.; Bovee, T.F.; Rietjens, I.M. Marine neurotoxins: State of the art, bottlenecks, and perspectives for mode of action based methods of detection in seafood. Mol. Nutr. Food Res. 2014, 58, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, A.; Eixarch, H.; de la Iglesia, P.; Rodriguez, M.; Dominguez, L.; Andree, K.B.; Diogène, J. Towards the standardisation of the neuroblastoma (neuro-2a) cell-based assay for ciguatoxin-like toxicity detection in fish: Application to fish caught in the Canary Islands. Food Addit. Contam. 2012, 29, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef] [PubMed]
- Amzil, Z.; Sibat, M.; Royer, F.; Masson, N.; Abadie, E. Report on the first detection of pectenotoxin-2, spirolide-a and their derivatives in French shellfish. Mar. Drugs 2007, 5, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Amzil, Z.; Sibat, M.; Royer, F.; Savar, V. First report on azaspiracid and yessotoxin groups detection in French shellfish. Toxicon 2008, 52, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; Abadie, E.; Hervé, F.; Berteaux, T.; Séchet, V.; Aráoz, R.; Molgó, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from ingril, a french mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef] [PubMed]








| Site | (n) | Macroalgae | |
|---|---|---|---|
| Genus | Total 1 | ||
| Anaho | 8 | Turf, Halimeda | 1.5 ± 2.6 |
| Taipivai | 2 | Halimeda micronesica | 0 |
| Taiohae | - | NF 2 | - |
| Species | Location | ||
|---|---|---|---|
| Anaho Bay n = 6 | Taipivai n = 6 | Taiohae n = 6 | |
| G. caribaeus | <1% | <1% | <1% |
| G. carpenteri | 17% | 90% | 88% |
| G. pacificus | <1% | <1% | <1% |
| G. polynesiensis | 82% | 10% | 10% |
| G. toxicus | <1% | <1% | 1% |
| Total cells | ~2900 | ~415 | ~420 |
| Site | Gambierdiscus Species | |||
|---|---|---|---|---|
| n 1 | G. polynesiensis | G. carpenteri | G. pacificus | |
| Anaho | 11 | 0 | 11 | 0 |
| Taipivai | 2 | 0 | 1 | 1 |
| Taiohae | 4 | 1 | 1 | 2 |
| Fractions | Toxin Content 1 | ||
|---|---|---|---|
| July 2014 | December 2014 | November 2016 | |
| LF70/30 | 0.03 ± 0.01 | ND 2 | ND |
| LF90/10 | 6.63 ± 1.95 | 1.59 ± 0.11 | 0.67 ± 0.06 |
| LF100 | 4.63 ± 1.63 | 0.51 ± 0.05 | ND |
| HF50/50 | ND | ND | ND |
| HF70/30 | ND | ND | ND |
| HF90/10 | 0.07 ± 0.01 | 0.06 ± 0.01 | ND |
| HF100 | 0.10 ± 0.03 | ND | ND |
| Total toxin content | 11.47 ± 3.91 | 2.16 ± 0.17 | 0.67 ± 0.06 |
| Date | Fractions | P-CTX-3C | P-CTX-3B | P-CTX-4A | P-CTX-4B | Total |
|---|---|---|---|---|---|---|
| July 2014 | First Extract FE | 8.1 | 14.81 | <LD 1 | <LD | 23 |
| SPE Si Extract | 5.8 | 13.0 | 0.9 | 0.8 | 21 | |
| December 2014 | SPE Si Extract | 3.57 | 2.2 | <LD | <LD | 5.8 |
| November 2016 | SPE Si Extract | <LD | 1.16 | <LD | <LD | 1.16 |
| Species | Name of Primers | Sequences of qPCR Primers |
|---|---|---|
| G. australes | GaustF10 | 5′-ATTGCTGTGTGAATACAGGTAA-3′ |
| GaustR10 | 5′-CAAGCACTGCCCACAGATAC-3′ | |
| G. pacificus | GpacifITSF1 | 5′-AATTCGAAACAGATGTGCATGG-3′ |
| GpacifITSR1 | 5′-GCCAAAGACAGCACTGATGAC-3′ | |
| G. polynesiensis | PolyITSF1 | 5′-TGTGTGCACGTGTGTGTATGG-3′ |
| PolyITSR1 | 5′-CGCACCACCGGCGCACAG-3′ | |
| G. toxicus | GtoxITSF1 | 5′-TGAGACAGACGTGCATGGTTG-3′ |
| GtoxITSR1 | 5′-CCAACAGCAGCACTGATGAAT-3′ | |
| G. belizeanus | GbelizeF1 | 5′-TAGAGGAATTGACACAAACTTG-3′ |
| GbelizeR1 | 5′-CATCAGGGTTTTCAGGTCAAA-3′ | |
| G. caribaeus | GcaribF3 | 5′-TGTCTTTGACTGGATGACTGT-3′ |
| GcaribR8 | 5′-TGTCTCCAACATGCTGGCAC-3′ | |
| G. carpenteri | GcarpenteriF1 | 5′-GGTGCTGTTGTGTGACCATA-3′ |
| GcarpenteriR3 | 5′-CTGGCAGTGGAAGCTGACA-3′ | |
| G. carolinianus | GcarolinF2 | 5′-TAAATGAGAAAGGACGCAGC-3′ |
| GcarolinR5 | 5′-CACCTCTCACTTCAAATTGG-3′ | |
| F. ruetzleri | GrutzITSF3 | 5′-TGGATAACACCATGGGAAGTC-3′ |
| GrutzITSR4 | 5′-TTCCCAGCTTCGAGGGGAAA-3′ | |
| Gambierdiscus ribotype 2 | RiboII-F1 | 5′-TTGGAGAGTGAATCTTGTCTT-3′ |
| RiboII-R1 | 5′-CGGTATCTGGCTTTGCGTG-3′ |
| Island | Area | Date | n | Shell Size 1 Basal Diameter (mm) | Whole Flesh Weight (g) |
|---|---|---|---|---|---|
| Nuku Hiva | Anaho | July 2014 | 13 | 117.7 ± 7.0 | 57.63 ± 15.2 2 |
| December 2014 | 10 | 65.0 ± 8.8 | 38.2 ± 10.4 2 | ||
| November 2016 | 12 | 117.0 ± 5.0 | 87.8 ± 19.4 3 | ||
| Taipivai | December 2014 | 13 | 62.3 ± 7.6 | 36.1 ± 8.8 2 | |
| November 2016 | 10 | 121.0 ± 4.6 | 100.1 ± 7.4 3 | ||
| Taiohae | November 2016 | 12 | 115.0 ± 8.0 | 85.0 ± 16.9 3 |
| Compound | Precursor Ion (Q1) m/z | Product Ion (Q2) m/z | Collision Energy (CE, eV) | Collision Exit Potential (CXP, eV) | Retention Time (RT, min) |
|---|---|---|---|---|---|
| P-CTX1B | 1128.6 | 1093.6 | 20 | 12 | 2.9 |
| 1075.6 | 30 | 12 | |||
| P-CTX3C and P-CTX3B | 1040.6 | 1005.6 | 30 | 12 | 11.3 and 11.5 |
| 1023.6 | 1005.6 | 20 | 12 | ||
| P-CTX4A and P-CTX4B | 1078.6 | 1043.6 | 30 | 12 | 12.4 and 12.8 |
| 1061.6 | 1043.6 | 20 | 12 | ||
| 2,3-diOH-P-CTX3C | 1074.6 | 1057.6 | 30 | 12 | |
| 1057.6 | 1039.6 | 20 | 12 | ||
| 51-OH-P-CTX3C | 1056.6 | 1021.6 | 30 | 12 | 6.1 |
| 1039.6 | 1021.6 | 20 | 12 | ||
| M-seco-P-CTX3C | 1041.6 | 1023.6 | 30 | 12 | 4.6 |
| 1005.6 | 20 | 12 | |||
| P-CTX2 and P-CTX3 | 1112.6 | 1077.6 | 20 | 12 | |
| 1059.6 | 30 | 12 | |||
| 2-OH-P-CTX3C and 3-OH-P-CTX3C | 1058.6 | 1023.6 | 30 | 12 | 4.8 |
| 1005.6 | 20 | 12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darius, H.T.; Roué, M.; Sibat, M.; Viallon, J.; Gatti, C.M.i.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Detection of Pacific Ciguatoxins in Toxic Samples from Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 2. https://doi.org/10.3390/toxins10010002
Darius HT, Roué M, Sibat M, Viallon J, Gatti CMi, Vandersea MW, Tester PA, Litaker RW, Amzil Z, Hess P, et al. Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Detection of Pacific Ciguatoxins in Toxic Samples from Nuku Hiva Island (French Polynesia). Toxins. 2018; 10(1):2. https://doi.org/10.3390/toxins10010002
Chicago/Turabian StyleDarius, Hélène Taiana, Mélanie Roué, Manoella Sibat, Jérôme Viallon, Clémence Mahana iti Gatti, Mark W. Vandersea, Patricia A. Tester, R. Wayne Litaker, Zouher Amzil, Philipp Hess, and et al. 2018. "Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Detection of Pacific Ciguatoxins in Toxic Samples from Nuku Hiva Island (French Polynesia)" Toxins 10, no. 1: 2. https://doi.org/10.3390/toxins10010002
APA StyleDarius, H. T., Roué, M., Sibat, M., Viallon, J., Gatti, C. M. i., Vandersea, M. W., Tester, P. A., Litaker, R. W., Amzil, Z., Hess, P., & Chinain, M. (2018). Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Detection of Pacific Ciguatoxins in Toxic Samples from Nuku Hiva Island (French Polynesia). Toxins, 10(1), 2. https://doi.org/10.3390/toxins10010002