Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs
Abstract
:1. Introduction
2. Results
2.1. Effect of DON and ZEN Exposure on Pig Growth Performance
2.2. Effect of DON and ZEN Exposure on Hematological Variables
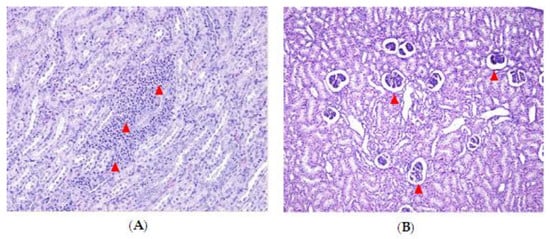
2.3. Effect of DON and ZEN Exposure on Histopathology
2.4. Effect of DON and ZEN Exposure on Immunological Variables
2.5. Effect of DON and ZEN Exposure on Total Antioxidant Capacity and Serotonin Levels
2.6. Effect of DON and ZEN on the Expression of Immune Response-Related Genes in Muscle, Liver, and Kidney
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Animal Exposure to DON and ZEN and Experimental Design
5.3. Mycotoxin Analysis
5.4. Growth Performance
5.5. Blood Collection
5.6. Tissues and Urine Collection
5.7. Histopathological Examination
5.8. Measurement of Total Immunoglobulin Subsets
5.9. Measurement of TAC Levels
5.10. Measurement of Serotonin Levels
5.11. RNA Isolation, Reverse Transcription, and Quantitative PCR (qPCR)
5.12. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Döll, S.; Dänicke, S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Munkvold, G.P. Mycotoxins in ethanol co-products: Modeling economic impacts on the livestock industry and management strategies. J. Agric. Food Chem. 2008, 56, 3900–3911. [Google Scholar] [CrossRef] [PubMed]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Blaney, B.J.; Spencer, R.A.; Dodman, R.L. Rejection by pigs of mouldy grain containing deoxynivalenol. Aust. Vet. J. 1985, 62, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Accensi, F.; Pinton, P.; Callu, P.; Abella-Bourges, N.; Guelfi, J.F.; Grosjean, F.; Oswald, I.P. Ingestion of low doses of deoxynivalenol does not affect hematological, biochemical, or immune responses of piglets. J. Anim. Sci. 2006, 84, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.V.L.N.; Smith, T.K.; MacDonald, E.J.; Boermans, H.J.; Squires, E.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2002, 80, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Bimczok, D.; Doll, S.; Rau, H.; Goyarts, T.; Wundrack, N.; Naumann, M.; Dänicke, S.; Rothkötter, H.J. The Fusarium toxin deoxynivalenol disrupts phenotype and function of monocyte-derived dendritic cells in vivo and in vitro. Immunobiology 2007, 212, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.L.; Brooks, K.; Pestka, J.J. In vitro effects of vomitoxin (deoxynivalenol) on T-cell interleukin production and IgA secretion. Food. Chem. Toxicol. 1994, 32, 617–625. [Google Scholar] [CrossRef]
- Pinton, P.; Accensi, F.; Beauchamp, E.; Cossalter, A.M.; Callu, P.; Grosjean, F.; Oswald, I.P. Effets de la Consommation D’aliment Naturellement Contamine par Deoxynivalenol (DON) sur la Reponse Vaccinale du Porc; Journees Recherche Porcine: Paris, France, 2006; pp. 399–406. [Google Scholar]
- Pinton, P.; Accensi, F.; Beauchamp, E.; Cossalter, A.M.; Callu, P.; Grosjean, F.; Oswald, I.P. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 2008, 177, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 53, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.; Ayed-Boussema, I.; Azqueta Oscoz, A.; Lopez Ade, C.; Bacha, H. The role ofoxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidativedna damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 94–302. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Ouanes, Z.; Hassen, W.; Baudrimont, I.; Creppy, E.; Bacha, H. Cytotoxicityinhibition of DNA and protein syntheses and oxidative damage in cultured cellsexposed to zearalenone. Toxicol. In Vitro 2004, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Tudor, D.S. Effects of zearalenone and its derivatives on the innate immune response in swine. Toxicon 2010, 56, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Manda, G.; Motiu, M.; Neagoe, I.; Dragomir, C.; Stancu, M.; Calin, L. Effects of zearalenone and its derivatives on porcine immune response. Toxicol. In Vitro 2011, 25, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Gerdes, R.G.; Underhill, K.L.; Rotter, B.A.; Jui, P.Y.; Trenholm, H.L. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.P.; Küchenmeister, U.; Jonas, L.; Kohlschein, P.; Pöhland, R.; Dänicke, S. Influence of graded levels of fusarium toxin-contaminated cereal grains in dietson selected enzymatic and histological parameters of liver in gilts. Food Chem. Toxicol. 2006, 44, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.P.; Jonas, L.; Pöhland, R.; Schneider, F.; Dänicke, S. Effects of diets with cereal grains contaminated by graded levels of two Fusarium toxins onselected immunological and histological parameters of spleen in gilts. J. Anim. Sci. 2006, 84, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Hong, S.-Y.; Jeon, M.-H.; An, J.-M.; Kim, S.-Y.; Kim, H.-Y.; Yoon, B.R.; Chung, S.H. Simultaneous determination of the levels of deoxynivalenol, 3-acetyldeoxynivalenol, and nivalenol in grain and feed samples from South Korea using a high-performance liquid chromatography-photodiode array detector. Appl. Biol. Chem. 2016, 59, 881–887. [Google Scholar] [CrossRef]
- Chang, H.; Kim, W.; Park, J.-H.; Kim, D.; Kim, C.-R.; Chung, S.; Lee, C. The occurrence of zearolenone in South Korean feedstuffs between 2009 and 2016. Toxins 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Shin, S.Y.; Park, C.S.; Kim, B.G. Effects of feeding barley naturally contaminated with Fusarium mycotoxins on growth performance, nutrient digestibility, and blood chemistry of gilts and growth recoveries by feeding a non-contaminated diet. Asian. Australas. J. Anim. Sci. 2015, 28, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Gremmels, J.F. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Liu, F.X.; Johnston, L.A.; Chi, F.; Wang, Y. Effect of purified zearolenone with or without modified montmorillonite on nutrient availability, genital organs and serum hormones in post-weaning piglets. Livest. Sci. 2012, 144, 110–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, R.; Liu, M.; Shi, B.; Shan, A. Use of modified halloysite nanotubes in the feed reduces the toxic effects of zearalenone on sow reproduction and piglet development. Theriogenology 2015, 83, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Bergsjø, B.; Langseth, W.; Nafstad, I.; Jansen, J.H.; Larsen, H.J. The effects of naturally deoxynivalenol-contaminated oats on the clinical condition, blood parameters, performance and carcass composition of growing pigs. Vet. Res. Commun. 1993, 17, 283–294. [Google Scholar]
- Chattopadhyay, P.; Pandey, A.; Goyary, D.; Chaurasia, A.; Singh, L.; Veer, V. Technetium-99m-labeled deoxynivalenol from Fusarium mycotoxin alters organ toxicity in BALB/c mice by oral and intravenous route. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 258–263. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, M.M.; Tan, B.E.; Yin, Y.L.; Li, T.J.; Xiao, D.F.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J. Anim. Sci. 2013, 91, 4772–4780. [Google Scholar] [CrossRef] [PubMed]
- Drochner, W.; Schollenberger, M.; Piepho, H.P.; Gotz, S.; Lauber, U.; Tafaj, M.; Klobasa, F.; Weiler, U.; Claus, R.; Steffl, M. Serum IgA-promoting effects induced by feed loads containing isolated deoxynivalenol (DON) in growing piglets. J. Toxicol. Environ. Health A 2004, 67, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; He, M.; Yan, H.K.; Deng, X.B. Influence of deoxynivalenol on hematologic indexes by different administration methods in pigs. China Anim. Husb. Vet. Med. 2010, 37, 47–50. [Google Scholar]
- Rotter, B.A.; Thompson, B.K.; Lessard, M.; Trenholm, H.L.; Tryphonas, H. Influence of low-level exposure to Fusarium mycotoxins on selected immunological and hematological parameters in young swine. Fundam. Appl. Toxicol. 1994, 23, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mortson, M. Effect of feed-Borne Fusarium Mycotoxins on Equine Gastric Ulcer Syndrome. Ph.D. Thesis, Animal and Poultry Science and Toxicology, Guelph, ON, Canada, December 2012. [Google Scholar]
- Goyarts, T.; Dänicke, S.; Tiemann, U.; Rothkötter, H.J. Effect of the Fusarium toxin deoxynivalenol (DON) on IgA, IgM and IgG concentrations and proliferation of porcine blood lymphocytes. Toxicol. In Vitro 2006, 20, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Klobasa, F.; Doll, S.; Ganter, M.; Flachowsky, G. Effects of graded levels of Fusarium toxin contaminated wheat in diets for fattening pigs on growth performance, nutrient digestibility, deoxynivalenol balance and clinical serum characteristics. Arch. Anim. Nutr. 2004, 58, 1–17. [Google Scholar] [CrossRef]
- Atroshi, F.; Rizzo, A.F.; Veijalainen, P.; Lindberg, L.A.; Honkanen-Buzalski, T.; Andersson, K.; Hirvi, T.; Saloniemi, H. The effect of dietary exposure to DON and T-2 toxin on host resistance and serum immunoglobulins of normal and mastitic mice. J. Anim. Physiol. Anim. Nutr. 1994, 71, 223–233. [Google Scholar] [CrossRef]
- Ren, Z.H.; Zhou, R.; Deng, J.L.; Zuo, Z.C.; Peng, X.; Wang, Y.C.; Wang, Y.; Yu, S.M.; Shen, L.H.; Cui, H.M.; Fang, J. Effects of the Fusarium toxin zearalenone (ZEA) and/or deoxynivalenol (DON) on the serum IgA, IgG and IgM levels in mice. Food Agric. Immunol. 2014, 25, 600–606. [Google Scholar] [CrossRef]
- Geisberger, R.; Lamers, M.; Achatz, G. The riddle of the dual expression of IgM and IgD. Immunology 2006, 118, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Wang, Y.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Wang, Y.; Peng, X.; Cui, H.M.; Shen, L.H. Deoxynivalenol induces apoptosis in chicken spleniclymphocytes via the reactive oxygen species-mediated mitochondrial pathway. Environ. Toxicol. Pharmacol. 2015, 1, 339–466. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.J.; Cools, R.; Crockett, M.J.; Sahakian, B.J. Mood state moderates the role of serotonin in cognitive biases. J. Psychopharmacol. 2010, 24, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Reiter, M.; Pfaffl, M.W.; Meyer, H.H.D.; Bauer, J.; Meyr, K.H.D. Expression of immune relevant genes in pigs under the influence of low doses of deoxynivalenol (DON). Mycotoxin Res. 2011, 27, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.C.; Gras, M.A.; Marin, D.E.; Israel-Roming, F.; Stancu, M.; Taranu, I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/NF-kappaB signalling molecules in pigs. Br. J. Nutr. 2014, 111, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; et al. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food Chem. Toxicol. 2015, 80, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediaators, MAPK signaling molecultes, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Bouaziz, C.; Golli-Bennour, E.E.; Ouanes, Z.; Bacha, H. Comparative study of toxic effects of zearalenone and its two major metabolites alpha-zearalenol and beta-zearalenol on cultured human Caco-2 cells. J. Biochem. Mol. Toxicol. 2009, 23, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Gazzah, A.C.; El Golli Bennour, E.; Bouaziz, C.; Abid, S.; Ladjimi, M.; Bacha, H. Sequential events of apoptosis induced by zearalenone in cultured hepatocarcinoma cells. Mycotoxin Res. 2010, 26, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, S.; Mc Neeley, D.F.; Moon, A. Mechanisms of nutrient modulation of the immune response. J. Alleergy Clin. Immunol. 2005, 115, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrition Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Lewczuk, B.; Przybylska-Gornowicz, B.; Gajecka, M.; Targońska, K.; Ziółkowska, N.; Prusik, M.; Gajęcki, M. Histological structure of duodenum in gilts receiving low doses of zearalenone and deoxynivalenol in feed. Exp. Toxicol. Pathol. 2016, 68, 157–166. [Google Scholar] [CrossRef] [PubMed]

| Item | Control | DON | ZEN | SEM | p-Value |
|---|---|---|---|---|---|
| Initial BW (kg) | 19.00 | 19.60 | 19.16 | 0.70 | 0.822 |
| Final BW (kg) | 43.50 a | 36.72 b | 42.68 a | 1.25 | 0.005 |
| ADG (kg/day) | 0.845 a | 0.590 b | 0.811 a | 0.03 | 0.0001 |
| ADFI (kg/day) | 1.41 | 1.31 | 1.38 | 0.07 | 0.536 |
| FCR kg/kg | 1.68 b | 2.25 a | 1.70 b | 0.11 | 0.005 |
| Liver ratio (%) | 3.22 | 3.42 | 3.49 | 0.18 | 0.501 |
| Kidneys ratio (%) | 1.22 | 1.30 | 1.31 | 0.08 | 0.761 |
| Item | Control | DON | ZEN | SEM | p-Value |
|---|---|---|---|---|---|
| WBC | 18.43 | 18.87 | 19.52 | 1.195 | 0.815 |
| RBC | 7.04 | 6.70 | 6.59 | 0.284 | 0.555 |
| HGB | 12.40 | 11.42 | 11.60 | 0.364 | 0.196 |
| HCT | 38.70 | 36.16 | 36.60 | 1.445 | 0.464 |
| MCV | 55.03 | 54.04 | 55.48 | 0.998 | 0.571 |
| MCH | 17.63 | 17.06 | 17.64 | 0.334 | 0.395 |
| MCHC | 32.08 | 31.62 | 31.80 | 0.404 | 0.738 |
| RDW-CV | 16.45 b | 18.42 a | 16.48 b | 0.417 | 0.008 |
| RDW-SD | 37.90 b | 41.66 a | 38.38 b | 0.811 | 0.013 |
| PLT | 187.25 | 225.20 | 330.40 | 49.147 | 0.147 |
| MPV | 7.58 | 7.58 | 7.54 | 0.23 | 0.991 |
| PDW | 16.05 | 15.86 | 15.96 | 0.181 | 0.771 |
| PCT | 0.14 | 0.17 | 0.25 | 0.037 | 0.155 |
| Item | Control | DON | ZEN | SEM | p-Value | |
|---|---|---|---|---|---|---|
| IgA (mg/mL) | 0.61 | 0.86 | 0.69 | 0.12 | 0.456 | |
| IgG (mg/mL) | 8.46 a | 5.97 c | 6.72 b | 0.15 | 0.0001 | |
| IgM (mg/mL) | 1.84 a | 1.08 c | 1.24 b | 0.03 | 0.0001 | |
| Serum | TAC (nmol) | 109.45 a | 88.24 b | 95.98 ab | 5.56 | 0.045 |
| Serotonin (μg/mL) | 0.57 | 0.38 | 0.35 | 0.07 | 0.110 | |
| Urine | TAC (nmol) | 2.36 b | 4.33 a | 2.40 b | 0.39 | 0.007 |
| Serotonin (μg/mL) | 0.08 b | 0.17 a | 0.16 a | 0.02 | 0.020 | |
| Tissue | Inflammatory Genes | Dietary Treatments | SEM | Pr > F | ||
|---|---|---|---|---|---|---|
| Control | DON | ZEN | ||||
| Muscle | IFN-γ | 1.09 b | 2.01 a | 0.91 b | 0.22 | 0.009 |
| IL6 | 1.04 b | 1.89 a | 0.50 b | 0.24 | 0.004 | |
| IL10 | 2.67 a | 1.28 b | 1.56 b | 0.27 | 0.011 | |
| IL12B | 4.60 ab | 6.11 a | 2.85 b | 0.78 | 0.032 | |
| TNF-α | 2.46 ab | 2.76 a | 1.87 b | 0.27 | 0.094 | |
| PTGS2 | 5.15 a | 5.65 a | 2.43 b | 0.60 | 0.005 | |
| CCL4 | 0.34 | 0.33 | 0.21 | 0.06 | 0.242 | |
| CCL2 | 0.04 a | 0.03 ab | 0.005 b | 0.01 | 0.103 | |
| CXCL10 | 3.42 | 2.93 | 1.90 | 1.69 | 0.813 | |
| CLDN3 | 0.002 | 0.004 | 0.001 | 0.01 | 0.121 | |
| Liver | IFN-γ | 4.43 ab | 3.94 a | 2.78 b | 1.02 | 0.036 |
| IL6 | 2.22 | 2.22 | 1.82 | 0.38 | 0.637 | |
| IL10 | 2.67 a | 1.95 ab | 1.28 b | 0.31 | 0.029 | |
| IL12B | 3.79 a | 3.49 a | 1.93 b | 0.49 | 0.045 | |
| TNF-α | 2.38 | 2.36 | 1.22 | 0.37 | 0.076 | |
| PTGS2 | 3.50 | 3.09 | 2.65 | 0.57 | 0.595 | |
| CCL4 | 0.17 a | 0.05 b | 0.07 b | 0.02 | 0.007 | |
| CCL2 | 0.17 | 0.09 | 0.16 | 0.03 | 0.168 | |
| CXCL10 | 2.22 | 1.02 | 0.95 | 0.61 | 0.295 | |
| CLDN3 | 0.32 a | 0.10 b | 0.11 b | 0.05 | 0.025 | |
| Kidney | IFN-γ | 1.84 | 1.75 | 1.84 | 0.38 | 0.684 |
| IL6 | 5.29 a | 2.48 b | 3.74 ab | 0.82 | 0.102 | |
| IL10 | 1.90 | 1.55 | 1.66 | 0.35 | 0.787 | |
| IL12B | 3.08 | 5.31 | 3.06 | 0.90 | 0.161 | |
| TNF-α | 2.20 | 2.02 | 1.81 | 0.32 | 0.699 | |
| PTGS2 | 2.48 | 1.68 | 2.61 | 0.65 | 0.545 | |
| CCL4 | 0.01 a | 0.006 b | 0.005 b | 0.00 | 0.028 | |
| CCL2 | 0.12 | 0.06 | 0.06 | 0.03 | 0.285 | |
| CXCL10 | 0.28 | 0.25 | 0.25 | 0.07 | 0.932 | |
| CLDN3 | 0.01 a | 0.005 b | 0.007 ab | 0.01 | 0.083 | |
| Item | Control Diet |
|---|---|
| Ingredients (%) | |
| Ground corn | 58.56 |
| Soybean meal (46% crude protein) | 14.00 |
| Extruded soybean meal | 12.00 |
| Whey powder (12% crude protein) | 7.00 |
| Fish meal | 3.45 |
| Soybean oil | 1.60 |
| l-Lysine·HCL (78%) | 0.43 |
| dl-Methionine (99%) | 0.14 |
| l-Threonine (99%) | 0.12 |
| Calcium hydrophosphate | 1.08 |
| Limestone | 0.60 |
| Choline chloride (50%) | 0.20 |
| Sodium chloride | 0.32 |
| Vitamin–trace mineral premix a | 0.50 |
| Calculated nutrients (%) | |
| Metabolizable energy (kcal/kg) | 3444 |
| Crude fiber | 2.29 |
| Crude protein | 20.78 |
| Lysine | 1.47 |
| Methionine | 0.49 |
| Calcium | 0.75 |
| Phosphorus | 0.45 |
| Gene Name | Accession Number | Sequence 5′ to 3′ | Product Size (bp) |
|---|---|---|---|
| IFN-γ | NM_213948 | F: GGTAGCTCTGGGAAACTGAATG R: CTGACTTCTCTTCCGCTTTCTT | 150 |
| IL6 | NM_214399 | F: AGACGGATGCTTCCAATCTG R: CAGCCTCGACATTTCCCTTAT | 134 |
| IL10 | NM_214041 | F: ATCAAGGAGCACGTGAACTC R: CCTCTCTTGGAGCTTGCTAAA | 144 |
| IL12B | NM_214013 | F: GAAGTACAGAGTGGAGTGTCAG R: TGATGAAGAAGCTGCTGGTATAG | 128 |
| TNF-α | NM_214022 | F: CCTACTGCACTTCGAGGTTATC R: GGCTTTGACATTGGCTACAAC | 145 |
| PTGS2 | NM_214321 | F: GATGGCCACGAGTACAACTATC R: AAGATTCCTACCACCAGCAAC | 129 |
| CLDN3 | NM_001160075 | F: TCATCGGCAGCAGCATTATC R: GAGAGTCGTACACTTTGCACTG | 105 |
| CXCL10 | NM_001008691 | F: CAAGGAATACCTCTCTCCAGAAC R: ATCTCAACATGTGGGCAAGA | 128 |
| CCL4 | NM_213779 | F: TCCTGCTGCTTCACATACAC R: TACTCCTGGACCCAGTCATC | 158 |
| CCL2 | NM_214214 | F: TCACCTGCTGCTATACACTTAC R: GGTTCTGCACAGATCTCCTT | 139 |
| GAPDH | AF017079 | F: GTCTGGAGAAACCTGCCAAATA R: CCCAGCATCAAAGGTAGAAGAG | 152 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, K.E.; Song, J.; Lee, H.-J.; Kim, M.; Kim, D.-W.; Jung, H.J.; Kim, B.; Lee, Y.; Yu, D.; Kim, D.-W.; et al. Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs. Toxins 2018, 10, 114. https://doi.org/10.3390/toxins10030114
Reddy KE, Song J, Lee H-J, Kim M, Kim D-W, Jung HJ, Kim B, Lee Y, Yu D, Kim D-W, et al. Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs. Toxins. 2018; 10(3):114. https://doi.org/10.3390/toxins10030114
Chicago/Turabian StyleReddy, Kondreddy Eswar, Jaeyong Song, Hyun-Jeong Lee, Minseok Kim, Dong-Wook Kim, Hyun Jung Jung, Bumseok Kim, Yookyung Lee, Dongjo Yu, Dong-Woon Kim, and et al. 2018. "Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs" Toxins 10, no. 3: 114. https://doi.org/10.3390/toxins10030114
APA StyleReddy, K. E., Song, J., Lee, H. -J., Kim, M., Kim, D. -W., Jung, H. J., Kim, B., Lee, Y., Yu, D., Kim, D. -W., Oh, Y. K., & Lee, S. D. (2018). Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs. Toxins, 10(3), 114. https://doi.org/10.3390/toxins10030114