An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of the Optimal T-2 Antigen
2.2. Regeneration of Sensor Chip
2.3. Selection of T-2 Antibody
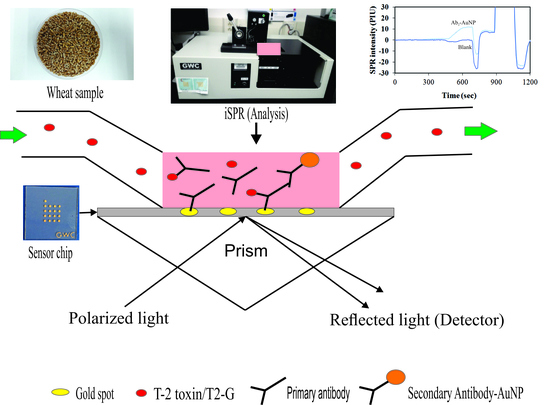
2.4. Effect of Ab2–AuNPs on the iSPR Assay
2.5. Response to T-2 Toxin and Cross-Reactivity with Analogs
2.6. Assay Performance
2.7. Establishment of Cutoff Levels for T-2 Toxin
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Wheat Samples
3.3. Preparation of Wheat Samples for the Validation Study
3.4. iSPR Biosensor
3.5. Optimization of Antigen–Antibody Interaction on the Sensor Chip
3.6. iSPR Assay Conditions
3.7. Cutoff Level Validation for T-2 Toxin
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ab1 | primary (antitoxin) antibody |
| Ab2–AuNPs | secondary antibody–gold nanoparticles |
| CDI | N, N’-carbonyldiimidazole |
| DON | deoxynivalenol |
| EC | European Commission |
| EDC | 1-ethyl-3 (3-dimetylaminopropyl) carbodiimide hydrochloride |
| EG | ethylene glycol |
| LOD | limit of detection |
| Mab | monoclonal antibody |
| PIU | pixel intensity unit |
| RS | regeneration solution |
| RSDr | relative standard deviation of repeatability |
| Sulfo-NHS | sulfo-N-hydroxysuccinimide |
| T2–BSA | T-2 bovine serum albumin |
| T2–CMO–BSA | T-2–carboxymethyloxime–BSA |
| T2G | T-2 glucoside |
| T2-G | T-2 toxin-3-glucoside |
| T2–OVA | T-2–ovalbumin |
References
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 2481, 1–187. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2011.2481/epdf (accessed on 24 January 2018).
- Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M.A. Fusarium langsethiae- a HT-2 and T-2 toxins producer that needs more attention. J. Phytopathol. 2012, 161, 1–10. [Google Scholar] [CrossRef]
- Biomin: Biomin Mycotoxin Survey. 2015. Available online: www.biomin.net (accessed on 24 October 2017).
- Rautala, T.; Hietaniemi, V.; Rämö, S.; Koivisto, T.; Ovaskainen, M.L.; Sinkko, H.; Kronberg-Kippilä, C.; Hirvonen, T.; Liukkonen, K.-H.; Kartio, M.; et al. Fusarium Toxins: Adult Intake from Cereals and Cereal-Based Products in Finland; Evira Research Reports 5; Finnish Food Safety Authority Evira: Helsinki, Finland, 2008; p. 44.
- SCF: Scientific Committee for Food. Opinion of the Scientific Committee for Food on Fusarium Toxins Part 5: T-2 Toxin and HT-2 Toxin. 2008. Available online: http://ec.europa.eu/food/fs/sc/scf/out88_en.pdf (accessed on 14 January 2018).
- Ueno, Y. General toxicity In Trichothecenes: Chemical, Biological and Toxicological Aspects; Ueno, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 135–146. [Google Scholar]
- McCormick, S.P.; Kato, T.; Maragos, C.M.; Busman, M.; Lattanzio, V.M.T.; Galaverna, G.; Dall-Asta, C.; Crich, D.; Price, N.P.; Kurtzman, C.P. Anomericity of T-2 toxin-glucoside: Masked mycotoxin in cereal crops. J. Agric. Food Chem. 2014, 63, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Van Poucke, C.; Wang, Z.; De Saeger, S.; De Boevre, M. Metabolic profile of the masked mycotoxin T-2 toxin-3-glucoside in rats (in vitro and in vivo) and humans (in vitro). World Mycotoxin J. 2017, 10, 349–362. [Google Scholar] [CrossRef]
- Berthiller, F.; DallAsta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass sepectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Busman, M.; Poling, S.M.; Maragos, C.M. Observation of T-2 toxin and HT-2 toxin glucosides from Fusarium sporotrichioides by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Toxins 2011, 3, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ohmichi, K.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H.; Yoshida, M.; Nakajima, T. Detection of a new Fusarium masked mycotoxin in wheat grain by high-resolution LC-Orbitrap MS. Food Addit. Contam. 2011, 28, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultenous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Ciasca, B.; Terzi, V.; Ghizzoni, R.; McCormick, S.P.; Pascale, M. Study of the natural occurrence of T-2 and HT-2 toxins and their glucosyl derivatives from field barley to malt by high-resolution orbitrap mass spectrometry. Food Addit. Contam. 2015, 32, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC) Recommendation 2013/165/EU on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products. Off. J. Eur. Union 2013, 91, 12–15. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0165&from=EN (accessed on 14 January 2018).
- Lattanzio, V.M.T.; Visconti, A.; Haidukowski, M.; Pascale, M. Identification and characterization of new Fusarium masked mycotoxins, T-2 and HT-2 glycosyl derivatives, in naturally contaminated wheat and oats by liquid chromatography-high resolution mass spectrometry. J. Mass Spectrom. 2012, 47, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Matsuo, Y. Development of an LC-MS/MS determination method for T-2 toxin and its glucoside and acetyl derivatives for estimating the contamination of total T-2 toxin in staple flours. J. AOAC Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, J.; Knoll, W. Biosensors based on surface plasmon enhanced flourescence Spectroscopy. Biointerphases 2008, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Reiner-Rozman, C.; Hageneder, S.; Dubiak-Szepietowska, M.; Dostalek, J.; Feller, K.H. Fast and sensitive detection of ochratoxin A in red wine by nano-particle-enhanced SPR. Anal. Chim. Acta 2016, 937, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, H.; Zhang, H.; He, G.; Li, X.; Zhang, X.; Liu, Y.; Li, C.M. Sensitive detection of multiple mycotoxins by SPRi with the gold nanoparticles as signal amplification tags. J. Colloid Interface Sci. 2014, 431, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Naimushin, A.N.; Soelberg, S.D.; Nguyen, D.K.; Dunlap, L.; Bartholomew, D.; Elkind, J.; Melendez, J.; Furlong, C.E. Detection of Staphylococcus aureus enterotoxin B at femtomolar levels with a miniature integrated two-chennel surface plasmon resonance (SPR) sensor. Bosens. Bioelectron. 2002, 17, 573–584. [Google Scholar] [CrossRef]
- Meneely, J.P.; Sulyok, M.; Baumgartner, S.; Krska, R.; Elliott, C.T. A rapid optical immunoassay for the screening of T-2 and HT-2 toxin in cereals and maize-based baby food. Talanta 2010, 81, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Segarra-Fas, A.; Peters, J.; Zuilhof, H.; van Beek, T.A.; Nielen, M.W.F. Multiplex surface plasma resonance biosensing and its transferability towards imaging nanoplasmonics for detection of mycotoxins in barley. Analyst 2016, 141, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Maragos, C.M. Gold nanoparticle-enhanced multiplexed imaging surface Plasmon resonance (iSPR) detection of Fusarium mycotoxins in wheat. Biosens. Bioelectron. 2018, 101, 245–252. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC) Regulation No 519/2014. Amending regulation (EC) No 401/2006 as regards methods of sampling of large lots, spies and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, 147, 29–43. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0519&from=EN (accessed on 6 December 2017).
- Maragos, C.M.; Kurtzman, C.; Busman, M.; Price, N.; McCormick, S.P. Development and evaluation of monoclonal antibodies for the glucoside of T-2 toxin (T2-Glc). Toxins 2013, 5, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and other biotransformations of T-2 toxin by yeasts of the Trichomonascus clade. Appl. Environ. Microbiol. 2012, 78, 5694–8702. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Clarke, J.R.; Marquardt, R.R.; Frohlich, A.A. Improved methods for conjugating selected mycotoxins to carrier proteins and dextran for immunoassays. J. Agric. Food Chem. 1995, 43, 2092–2097. [Google Scholar] [CrossRef]
- Zhang, G.S.; Schubring, S.L.; Chu, F.S. Improved method for production of antibodies against T-2 toxin and diacetoxyscirpenol in rabbits. Appl. Environ. Microbiol. 1986, 51, 132–137. [Google Scholar] [PubMed]
- Maragos, C.M. Signal amplification using colloidal gold in a biolayer interferometry-based immunosensor for the mycotoxin deoxynivalenol. Food Addit. Contam. 2012, 29, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Holst, C.V.; Visconti, A. Experimental design for in-house validation of a screen of immunoassay kit. The case of a mutiplex dipstick for Fusarium mycotoxins in creals. Anal. Bioanal. Chem. 2013, 405, 7773–7782. [Google Scholar] [CrossRef] [PubMed]






| Mycotoxins | IC50 (ng/mL) | Cross-Reactivity (%) |
|---|---|---|
| T-2 toxin | 5.17 | 100 |
| T2-G | 2.84 | 182 |
| DON | ND a | <1 |
| 3-acetyl-DON | ND | <1 |
| 15-acetyl-DON | ND | <1 |
| HT-2 toxin | ND | <1 |
| HT-2 toxin glucoside | ND | <1 |
| Nivalenol | ND | <1 |
| Spiking Levels (µg/kg) | Recovery (%) | |
|---|---|---|
| T-2 Toxin ± RSDr | T2-G ± RSDr | |
| 50 | 84 ± 5% | 102 ± 2% |
| 100 | 80 ± 6% | 66 ± 4% |
| 200 | --- | 102 ± 12% |
| 500 | 93 ± 2% | --- |
| Average | 86 ± 4% | 90 ± 6% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.Z.; McCormick, S.P.; Maragos, C.M. An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat. Toxins 2018, 10, 119. https://doi.org/10.3390/toxins10030119
Hossain MZ, McCormick SP, Maragos CM. An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat. Toxins. 2018; 10(3):119. https://doi.org/10.3390/toxins10030119
Chicago/Turabian StyleHossain, Md Zakir, Susan P. McCormick, and Chris M. Maragos. 2018. "An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat" Toxins 10, no. 3: 119. https://doi.org/10.3390/toxins10030119
APA StyleHossain, M. Z., McCormick, S. P., & Maragos, C. M. (2018). An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat. Toxins, 10(3), 119. https://doi.org/10.3390/toxins10030119