Interaction between Insects, Toxins, and Bacteria: Have We Been Wrong So Far?
Abstract
:1. Introduction
2. A Historical Dichotomy
3. When Semantic Misleads More Than It Informs
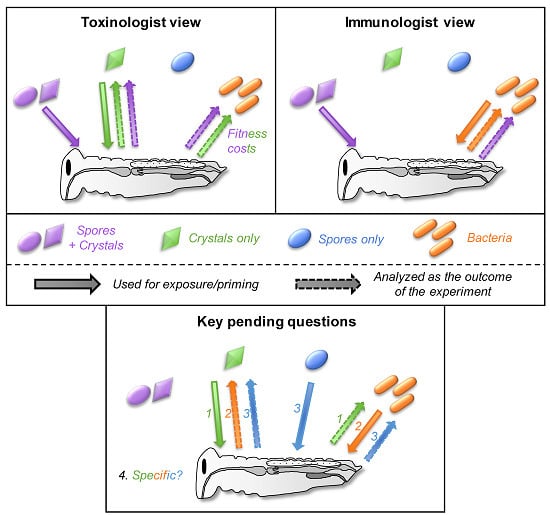
4. A Prerequisite: Disentangle Confounding Effects
4.1. Trans-Generational Immune Priming (TGIP) Versus Evolved Bacterial Resistance
4.2. One-Toxin Versus Multi-Toxins Resistance
5. Cross-Effects between Toxins, Bacteria, and Spores
5.1. Can Toxins Trigger an Immune Response in the Host and Lead to the Protection against Bacteria?
5.2. Is the Immune System Involved in the Response to Toxins?
5.3. Do Spores Play an Unsuspected Major Role in the Response to Bt Toxins and Bacteria?
5.4. How Specific Are These Cross-effects between Toxins, Bacteria, and Spores?
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Henkel, J.S.; Baldwin, M.R.; Barbieri, J.T. Toxins from Bacteria. EXS 2010, 100, 1–29. [Google Scholar] [PubMed]
- Schmitt, C.K.; Meysick, K.C.; O’Brien, A.D. Bacterial Toxins: Friends or Foes? Emerg. Infect. Dis. 1999, 5, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudkin, J.K.; McLoughlin, R.M.; Preston, A.; Massey, R.C. Bacterial toxins: Offensive, defensive, or something else altogether? PLoS Pathog. 2017, 13, e1006452. [Google Scholar] [CrossRef] [PubMed]
- Jamet, A.; Charbit, A.; Nassif, X. Antibacterial Toxins: Gram-Positive Bacteria Strike Back! Trends Microbiol. 2018, 26, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S. Evolution of host resistance to insect pathogens. Curr. Opin. Insect Sci. 2017, 21, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ash, G.J. Whole genome phylogeny of Bacillus by feature frequency profiles (FFP). Sci. Rep. 2015, 5, 13644. [Google Scholar] [CrossRef]
- Rasko, D.A.; Altherr, M.R.; Han, C.S.; Ravel, J. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 2005, 29, 303–329. [Google Scholar] [PubMed]
- Vachon, V.; Laprade, R.; Schwartz, J.L. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J. Invertebr. Pathol. 2012, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, B.; Hofling, C.; Futo, M.; Scharsack, J.P.; Kurtz, J. Infection of Tribolium castaneum with Bacillus thuringiensis: Quantification of bacterial replication within cadavers, transmission via cannibalism, and inhibition of spore germination. Appl. Environ. Microbiol. 2015, 81, 8135–8144. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Bonsall, M.B. Cooperation and the evolutionary ecology of bacterial virulence: The Bacillus cereus group as a novel study system. BioEssays 2013, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Federici, B.A.; Park, H.W.; Bideshi, D.K.; Wirth, M.C.; Johnson, J.J. Recombinant bacteria for mosquito control. J. Exp. Biol. 2003, 206, 3877–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabashnik, B.E.; Huang, F.N.; Ghimire, M.N.; Leonard, B.R.; Siegfried, B.D.; Rangasamy, M.; Yang, Y.J.; Wu, Y.D.; Gahan, L.J.; Heckel, D.G.; et al. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat. Biotechnol. 2011, 29, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Brevault, T.; Carriere, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y. Insecticide Resistance. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: New York, NY, USA, 2008; Volume 1, pp. 1979–1980. [Google Scholar]
- Horns, F.; Hood, M.E. The evolution of disease resistance and tolerance in spatially structured populations. Ecol. Evol. 2012, 2, 1705–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Garcia, M.; Conde, R.; Bello-Bedoy, R.; Lanz-Mendoza, H. The damage threshold hypothesis and the immune strategies of insects. Infect. Genet. Evol. 2014, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG transplacental transfer on early life immunity. ImmunoHorizons 2018, 2, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Hasselquist, D.; Nilsson, J.A. Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philos. Trans. Royal Soc. Lond. Ser. B Biol. Sci. 2009, 364, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Son, Y.L. Maternal transmission of immunity to white spot syndrome associated virus (WSSV) in shrimp (Penaeus monodon). Dev. Compara. Immunol. 1999, 23, 545–552. [Google Scholar] [CrossRef]
- Pigeault, R.; Garnier, R.; Rivero, A.; Gandon, S. Evolution of transgenerational immunity in invertebrates. Proc. Biol. Sci. 2016, 283, 20161136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubuffet, A.; Zanchi, C.; Boutet, G.; Moreau, J.; Teixeira, M.; Moret, Y. Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle. PLoS Pathog. 2015, 11, e1005178. [Google Scholar] [CrossRef] [PubMed]
- Dhinaut, J.; Chogne, M.; Moret, Y. Trans-generational immune priming in the mealworm beetle protects eggs through pathogen-dependent mechanisms imposing no immediate fitness cost for the offspring. Dev. Compara. Immunol. 2018, 79, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. The role of epigenetics in host-parasite coevolution: lessons from the model host insects Galleria Mellon. Tribol. Castaneum Zool. 2016, 119, 273–280. [Google Scholar]
- Salmela, H.; Amdam, G.V.; Freitak, D. Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin. PLoS Pathog. 2015, 11, e1005015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carriere, Y.; Crickmore, N.; Tabashnik, B.E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 2015, 33, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E. Delaying insect resistance to transgenic crops. Proc. Nat. Acad. Sci. USA 2008, 105, 19029–19030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carriere, Y.; Fabrick, J.A.; Tabashnik, B.E. Can Pyramids and Seed Mixtures Delay Resistance to Bt Crops? Trends Biotechnol. 2016, 34, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kain, W.; Song, X.; Janmaat, A.F.; Zhao, J.Z.; Myers, J.; Shelton, A.M.; Wang, P. Resistance of Trichoplusia ni populations selected by Bacillus thuringiensis sprays to cotton plants expressing pyramided Bacillus thuringiensis toxins Cry1Ac and Cry2Ab. Appl. Environ. Microbiol. 2015, 81, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Unnithan, G.C.; Yelich, A.J.; DeGain, B.; Masson, L.; Zhang, J.; Carriere, Y.; Tabashnik, B.E. Multi-Toxin Resistance Enables Pink Bollworm Survival on Pyramided Bt Cotton. Sci. Rep. 2015, 5, 16554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Amaya, O.F.; Rodrigues, J.V.; Souza, T.C.; Tavares, C.S.; Campos, S.O.; Guedes, R.N.; Pereira, E.J. Resistance to dual-gene Bt maize in Spodoptera frugiperda: Selection, inheritance, and cross-resistance to other transgenic events. Sci. Rep. 2015, 5, 18243. [Google Scholar] [CrossRef] [PubMed]
- Stalinski, R.; Laporte, F.; Despres, L.; Tetreau, G. Alkaline phosphatases are involved in the response of Aedes aegypti larvae to intoxication with Bacillus thuringiensis subsp. israelensis Cry toxins. Environ. Microbiol. 2016, 18, 1022–1036. [Google Scholar] [PubMed]
- Stalinski, R.; Laporte, F.; Tetreau, G.; Despres, L. Receptors are affected by selection with each Bacillus thuringiensis israelensis Cry toxin but not with the full Bti mixture in Aedes aegypti. Infect. Genet. Evol.: J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 44, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Wang, R.; Wang, P. Fitness of Bt-resistant cabbage loopers on Bt cotton plants. Plant Biotechnol. J. 2017, 15, 1322–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ericsson, J.D.; Janmaat, A.F.; Lowenberger, C.; Myers, J.H. Is decreased generalized immunity a cost of Bt resistance in cabbage loopers Trichoplusia ni? J. Invertebr. Pathol. 2009, 100, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, R.M.; Cervantes, V.; Myers, J.H. Resistance to Bacillus thuringiensis in the cabbage looper (Trichoplusia ni) increases susceptibility to a nucleopolyhedrovirus. J. Invertebr. Pathol. 2010, 105, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Despres, L.; Stalinski, R.; Tetreau, G.; Paris, M.; Bonin, A.; Navratil, V.; Reynaud, S.; David, J.P. Gene expression patterns and sequence polymorphisms associated with mosquito resistance to Bacillus thuringiensis israelensis toxins. BMC Genom. 2014, 15, 926. [Google Scholar] [CrossRef] [PubMed]
- Crava, C.M.; Jakubowska, A.K.; Escriche, B.; Herrero, S.; Bel, Y. Dissimilar Regulation of Antimicrobial Proteins in the Midgut of Spodoptera exigua Larvae Challenged with Bacillus thuringiensis Toxins or Baculovirus. PLoS One 2015, 10, e0125991. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Benito-Jardon, M.; Lopez-Galiano, M.J.; Real, M.D.; Rausell, C. Tribolium castaneum immune defense genes are differentially expressed in response to Bacillus thuringiensis toxins sharing common receptor molecules and exhibiting disparate toxicity. Dev. Compara. Immunol. 2015, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Rausell, C.; Real, M.D. Tribolium castaneum Apolipophorin-III acts as an immune response protein against Bacillus thuringiensis Cry3Ba toxic activity. J. Invertebr. Pathol. 2013, 113, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Martinez, P.; Gomis-Cebolla, J.; Ferre, J.; Escriche, B. Changes in gene expression and apoptotic response in Spodoptera exigua larvae exposed to sublethal concentrations of Vip3 insecticidal proteins. Sci. Rep. 2017, 7, 16245. [Google Scholar] [CrossRef] [PubMed]
- Anantanawat, K.J.; Glatz, R.; Keller, M.A. Effect of induced tolerance to Bt toxin on the egg size of Helicoverpa armigera and parasitism by Trichogramma pretiosum. Physiol. Entomol. 2016, 41, 267–273. [Google Scholar] [CrossRef]
- Rahman, M.M.; Roberts, H.L.S.; Sarjan, M.; Asgari, S.; Schmidt, O. Induction and transmission of Bacillus thuringiensis tolerance in the flour moth Ephestia kuehniella. Proc. Nat. Acad. Sci. USA 2004, 101, 2696–2699. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Roberts, H.; Sarjan, M.; Featherstone, N.; Lahnstein, J.; Akhurst, R.; Schmidt, O. Is the mature endotoxin Cry1Ac from Bacillus thuringiensis inactivated by a coagulation reaction in the gut lumen of resistant, Helicoverpa armigera larvae? Insect Biochem Mol. Biol. 2005, 35, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y. “Trans-generational immune priming”: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. Biol. Sci. 2006, 273, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Mylonakis, E. Effector triggered immunity. Virulence 2014, 5, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Gordon, O.; Ahrens, S.; Franz, A.; Debbouche, S.; Chakravarty, P.; Phillips, D.; Yunus, A.A.; Rosen, M.K.; Valente, R.S.; et al. Actin is an evolutionarily-conserved damage-associated molecular pattern that signals tissue injury in Drosophila melanogaster. eLIFE 2016, 5, e19662. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Berisha, A.; Mukherjee, K.; Spengler, B.; Rompp, A.; Vilcinskas, A. Identification of collagen IV derived danger/alarm signals in insect immunity by nanoLC-FTICR MS. Biol. Chem. 2009, 390, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, S.L.; Dong, Y.; Pike, A.; Blumberg, B.J.; Bahia, A.C.; Dimopoulos, G. Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium Antagonist. PLoS Pathog. 2015, 11, e1004631. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Pinaud, S.; Portet, A.; Galinier, R.; Gourbal, B.; Duval, D. Specific pathogen recognition by multiple innate immune sensors in an invertebrate. Front. Immunol. 2017, 8, 1249. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, danger and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Pradeu, T.; Cooper, E.L. The danger theory: 20 years later. Front. Immunol. 2012, 3, 287. [Google Scholar] [CrossRef] [PubMed]
- Masri, L.; Branca, A.; Sheppard, A.E.; Papkou, A.; Laehnemann, D.; Guenther, P.S.; Prahl, S.; Saebelfeld, M.; Hollensteiner, J.; Liesegang, H.; et al. Host-Pathogen Coevolution: The Selective Advantage of Bacillus thuringiensis Virulence and Its Cry Toxin Genes. PLoS Biol. 2015, 13, e1002169. [Google Scholar] [CrossRef] [PubMed]
- Kieser, K.J.; Kagan, J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017, 17, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Sastalla, I.; Leppla, S.H. Anthrax and the inflammasome. Microbes Infect. 2012, 14, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, S.; Peuss, R.; Milutinovic, B.; Eggert, H.; Esser, D.; Rosenstiel, P.; Schulenburg, H.; Bornberg-Bauer, E.; Kurtz, J. Infection routes matter in population-specific responses of the red flour beetle to the entomopathogen Bacillus thuringiensis. BMC Genom. 2014, 15, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Roberts, H.L.S.; Schmidt, O. Tolerance to Bacillus thuringiensis endotoxin in immune-suppressed larvae of the flour moth Ephestia kuehniella. J. Invertebr. Pathol. 2007, 96, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.M. Revealing the Achilles heel of bacterial toxins. Immunity 2014, 41, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, E.; Seveau, S.M.; Kudryashov, D.S. Targeting and inactivation of bacterial toxins by human defensins. Biol. Chem. 2017, 398, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Pechine, S.; Collignon, A. Immune responses induced by Clostridium difficile. Anaerobe 2016, 41, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte Gálvez, J.A.; Kelly, C.P. Bezlotoxumab: anti-toxin B monoclonal antibody to prevent recurrence of Clostridium difficile infection. Expert Rev. Gastroent. Hepatol. 2017, 11, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A. Sporulation and Delta-Endotoxin Synthesis by Bacillus Thuringiensis. Cell. Mol. Life Sci. 2002, 59, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, B.; Huang, W.; Dobens, L.; Song, H.; Ling, E. Gut immunity in Lepidopteran insects. Dev Compara. Immunol 2016, 64, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Lereclus, D.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Li, R.S.; Jarrett, P.; Burges, H.D. Importance of spores, crystals, and delta-endotoxins in the pathogenicity of different varieties of Bacillus thuringiensis in Galleria mellonella and Pieris brassicae. J. Invertebr. Pathol. 1987, 50, 277–284. [Google Scholar] [CrossRef]
- Johnson, D.E.; Oppert, B.; McGaughey, W.H. Spore coat protein synergizes Bacillus thuringiensis crystal toxicity for the indianmeal moth (Plodia interpunctella). Curr. Microbiol. 1998, 36, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.I.; Tyrell, D.J.; Fitz-James, P.C.; Bulla, L.A. Relationship of the syntheses of spore coat protein and parasporal crystal protein in Bacillus thuringiensis. J. Bacteriol. 1982, 151, 399–410. [Google Scholar] [PubMed]
- Delafield, F.P.; Somerville, H.J.; Rittenberg, S.C. Immunological homology between crystal and spore protein of Bacillus thuringiensis. J. Bacteriol. 1968, 96, 713–720. [Google Scholar] [PubMed]
- Du, C.; Nickerson, K.W. Bacillus thuringiensis HD-73 Spores Have Surface-Localized Cry1Ac Toxin: Physiological and Pathogenic Consequences. Appl. Environ. Microbiol. 1996, 62, 3722–3726. [Google Scholar] [PubMed]
- Short, J.A.; Walker, P.D.; Thomson, R.O.; Somerville, H.J. The fine structure of Bacillus finitimus and Bacillus thuringiensis spores with special reference to the location of crystal antigen. Anglais 1974, 84, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Johnston, P.R.; Wright, D.J.; Ellis, R.J.; Crickmore, N.; Bonsall, M.B. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ. Microbiol. 2009, 11, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raffa, K.F.; Handelsman, J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Nat. Acad. Sci. USA 2006, 103, 15196–15199. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Grizard, S.; Patil, C.D.; Tran, F.H.; Tran Van, V.; Stalinski, R.; Laporte, F.; Mavingui, P.; Despres, L.; Valiente Moro, C. Bacterial microbiota of Aedes aegypti mosquito larvae is altered by intoxication with Bacillus thuringiensis israelensis. Parasites Vectors 2018, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Wilcks, A.; Hansen, B.M.; Hendriksen, N.B.; Licht, T.R. Persistence of Bacillus thuringiensis bioinsecticides in the gut of human-flora-associated rats. FEMS Immunol. Med. Microbiol. 2006, 48, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Wilcks, A.; Hansen, B.M.; Hendriksen, N.B.; Licht, T.R. Fate and effect of ingested Bacillus cereus spores and vegetative cells in the intestinal tract of human-flora-associated rats. FEMS Immunol. Med. Microbiol. 2006, 46, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Futo, M.; Armitage, S.A.; Kurtz, J. Microbiota Plays a Role in Oral Immune Priming in Tribolium castaneum. Front. Microbiol. 2015, 6, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futo, M.; Sell, M.P.; Kutzer, M.A.M.; Kurtz, J. Specificity of oral immune priming in the red flour beetle Tribolium castaneum. Biol. Lett. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Roth, O.; Sadd, B.M.; Schmid-Hempel, P.; Kurtz, J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. Biol. Sci. 2009, 276, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetreau, G. Interaction between Insects, Toxins, and Bacteria: Have We Been Wrong So Far? Toxins 2018, 10, 281. https://doi.org/10.3390/toxins10070281
Tetreau G. Interaction between Insects, Toxins, and Bacteria: Have We Been Wrong So Far? Toxins. 2018; 10(7):281. https://doi.org/10.3390/toxins10070281
Chicago/Turabian StyleTetreau, Guillaume. 2018. "Interaction between Insects, Toxins, and Bacteria: Have We Been Wrong So Far?" Toxins 10, no. 7: 281. https://doi.org/10.3390/toxins10070281
APA StyleTetreau, G. (2018). Interaction between Insects, Toxins, and Bacteria: Have We Been Wrong So Far? Toxins, 10(7), 281. https://doi.org/10.3390/toxins10070281