An Automated and High-Throughput Immunoaffinity Magnetic Bead-Based Sample Clean-Up Platform for the Determination of Aflatoxins in Grains and Oils Using UPLC-FLD
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of IMB
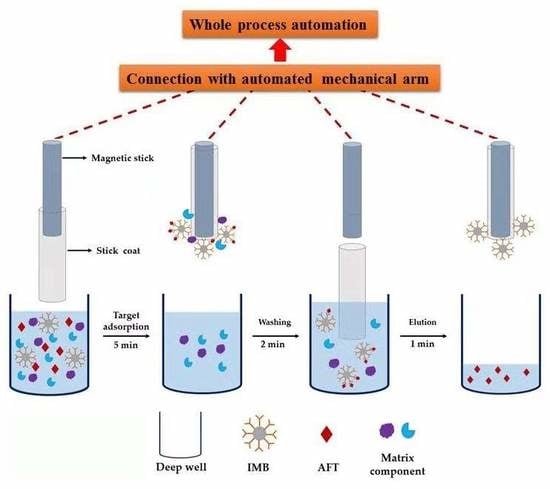
2.2. Automated Clean-up Platform
2.3. Optimization of the Sample Clean-up Method
2.4. Development of Clean-up of IMB Coupled to UPLC–FLD Detection
2.5. Method Validation of Clean-Up of IMB Coupled to UPLC–FLD Detection
2.5.1. Linearity and Sensitivity
2.5.2. Accuracy and Precision
2.5.3. Comparison between the IMB Automated Clean-up Method and the Conventional IAC Clean-up Method
2.6. Interlaboratory Study
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of IMB
4.3. Characterization of IMB
4.4. Sample Extraction
4.5. Automated IMB Clean-Up System
4.6. Automated IMB Clean-up Procedure and Optimization.
4.7. Manual IAC Clean-up Procedure
4.8. UPLC-FLD Analysis
4.9. Validation of Analytical Method
4.10. Interlaboratory Study
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; Zhang, D.; Guan, D.; Liu, D.X.; Fang, S.; Wang, X. Aflatoxin Measurement and Analysis. In Aflatoxins—Detection, Measurement and Control; InTech: Rijeka, Croatia, 2011; pp. 183–203. [Google Scholar]
- Sun, D.; Qiu, N.; Zhou, S.; Lyu, B.; Zhang, S.; Li, J.; Zhao, Y.; Wu, Y. Development of Sensitive and Reliable UPLC-MS/MS Methods for Food Analysis of Emerging Mycotoxins in China Total Diet Study. Toxins 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luan, Y.; Lu, A.; Li, B.; Yang, M.; Wang, J. An oligosorbent-based aptamer affinity column for selective extraction of aflatoxin B-2 prior to HPLC with fluorometric detection. Microchim. Acta 2018, 185, 71. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Rastrelli, L. Application of dispersive liquid-liquid microextraction for the determination of aflatoxins B-1, B-2, G(1) and G(2) in cereal products. J. Chromatogr. A 2011, 1218, 7648–7654. [Google Scholar] [CrossRef] [PubMed]
- Simao, V.; Merib, J.; Dias, A.N.; Carasek, E. Novel analytical procedure using a combination of hollow fiber supported liquid membrane and dispersive liquid-liquid microextraction for the determination of aflatoxins in soybean juice by high performance liquid chromatography—Fluorescence detector. Food Chem. 2016, 196, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Miro-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERS and liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 229, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Chu, X.; Ling, Y.; Huang, J.; Chang, J. Multi-mycotoxin analysis in dairy products by liquid chromatography coupled to quadrupole orbitrap mass spectrometry. J. Chromatogr. A 2014, 1345, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Z.; Hu, X.; Zhang, Q. Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects. Mass Spectrom. Rev. 2013, 32, 420–452. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, Y.; Guo, Q.; Lu, M.; Wang, S.; Xin, Y.; Xie, G.; Zhang, Y.; Mariappan, M.; Wang, S. Development and Interlaboratory Study of a Liquid Chromatography Tandem Mass Spectrometric Methodfor the Determination of Multiple Mycotoxins inCereals Using Stable Isotope Dilution. J. AOAC Int. 2018, 101, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Schaab, M.R.; Southwood, G.; Tor, E.R.; Aston, L.S.; Song, W.; Eitzer, B.; Majumdar, S.; Lapainis, T.; Mai, H.; et al. A Collaborative Study: Determination of Mycotoxins in Corn, Peanut Butter, and Wheat Flour Using Stable Isotope Dilution Assay (SIDA) and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J. Agric. Food Chem. 2017, 65, 7138–7152. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Hu, X.; Zhang, Q.; Li, P. Determination for multiple mycotoxins in agricultural products using HPLC-MS/MS via a multiple antibody immunoaffinity column. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1021, 145–152. [Google Scholar] [CrossRef]
- Senyuva, H.Z.; Gilbert, J. Immunoaffinity column clean-up techniques in food analysis: A review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Pezzi, H.M.; Niles, D.J.; Schehr, J.L.; Beebe, D.J.; Lang, J.M. Integration of Magnetic Bead-Based Cell Selection into Complex Isolations. ACS Omega 2018, 3, 3908–3917. [Google Scholar] [CrossRef] [Green Version]
- Schneck, N.A.; Phinney, K.W.; Lee, S.B.; Lowenthal, M.S. Quantification of antibody coupled to magnetic particles by targeted mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 8325–8332. [Google Scholar] [CrossRef]
- Sacligil, D.; Senel, S.; Yavuz, H.; Denizli, A. Purification of transferrin by magnetic immunoaffinity beads. J. Sep. Sci. 2015, 38, 2729–2736. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L. Magnetic Materials for the Selective Analysis of Peptide and Protein Biomarkers. Curr. Med. Chem. 2017, 24, 438–453. [Google Scholar] [CrossRef]
- Zhao, L.; Whiteaker, J.R.; Voytovich, U.J.; Ivey, R.G.; Paulovich, A.G. Antibody-Coupled Magnetic Beads Can Be Reused in Immuno-MRM Assays To Reduce Cost and Extend Antibody Supply. J. Proteome Res. 2015, 14, 4425–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palandra, J.; Quazi, A.; Fitz, L.; Rong, H.; Morris, C.; Neubert, H. Quantitative measurements of GDF-8 using immunoaffinity LC-MS/MS. Proteom. Clin. Appl. 2016, 10, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Pero-Gascon, R.; Pont, L.; Benavente, F.; Barbosa, J.; Sanz-Nebot, V. Analysis of serum transthyretin by on-line immunoaffinity solid-phase extraction capillary electrophoresis mass spectrometry using magnetic beads. Electrophoresis 2016, 37, 1220–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pont, L.; Benavente, F.; Barbosa, J.; Sanz-Nebot, V. On-line immunoaffinity solid-phase extraction capillary electrophoresis mass spectrometry using Fab antibody fragments for the analysis of serum transthyretin. Talanta 2017, 170, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Todoroki, K.; Nakano, T.; Eda, Y.; Ohyama, K.; Hayashi, H.; Tsuji, D.; Min, J.Z.; Inoue, K.; Iwamoto, N.; Kawakami, A.; et al. Bioanalysis of bevacizumab and infliximab by high-temperature reversed-phase liquid chromatography with fluorescence detection after immunoaffinity magnetic purification. Anal. Chim. Acta 2016, 916, 112–119. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhu, J.; Nie, Y.; Hu, R.; Wang, T.; Li, P.; Zhang, Q.; Yang, Y. Nanobody Technology for Mycotoxin Detection: Current Status and Prospects. Toxins 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Lai, W.; Saini, J.; Shan, S.; Cui, X.; Liu, D. Rapid pretreatment and detection of trace aflatoxin B1 in traditional soybean sauce. Food Chem. 2014, 150, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z.; Zhao, M.; Lau, S.C.S.; Tan, H.R.; Teh, W.J.; Yang, H.; Zheng, C.; Zhang, Y. Quantification of aflatoxin B1 in vegetable oils using low temperature clean-up followed by immuno-magnetic solid phase extraction. Food Chem. 2019, 275, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, A.; Fu, H.; Li, B.; Yang, M.; Wang, J.; Luan, Y. Affinity capture of aflatoxin B-1 and B-2 by aptamer-functionalized magnetic agarose microspheres prior to their determination by HPLC. Microchim. Acta 2018, 185, 326. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guan, Y.; Liu, H. Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3433–3439. [Google Scholar] [CrossRef]
- European Commission Regulation (EC). No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- ISO. 5725-2: Accuracy (Trueness and Precision) of Measurement Methods and Results-Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Horwitz, W.; Albert, R. The Horwitz ratio (HorRat): A useful index of method performance with respect to precision. J. AOAC Int. 2006, 89, 1095–1109. [Google Scholar] [PubMed]
- Ye, J.; Xuan, Z.; Zhang, B.; Wu, Y.; Li, L.; Wang, S.; Xie, G.; Wang, S. Automated analysis of ochratoxin A in cereals and oil by immunoaffinity magnetic beads coupled to UPLC-FLD. Food Control 2019, 104, 57–62. [Google Scholar] [CrossRef]
- Bao, L.; Liang, C.; Trucksess, M.W.; Xu, Y.; Lv, N.; Wu, Z.; Jing, P.; Fry, F.S. Determination of Aflatoxins B-1, B-2, G(1), and G(2) in Olive Oil, Peanut Oil, and Sesame Oil Using Immunoaffinity Column Cleanup, Postcolumn Derivatization, and Liquid Chromatography with Fluorescence Detection: First Action 2013.05. J. AOAC Int. 2013, 96, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ellison, S.L.R.; Wood, R. The International Harmonized Protocol for the proficiency testing of analytical chemistry laboratories (IUPAC technical report). Pure Appl. Chem. 2006, 78, 145–196. [Google Scholar] [CrossRef]



| Maize | Wheat | Husked Rice | Peanut oil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiking Levels, μg/kg | Recovery, % | RSD, % | Spiking Levels, μg/kg | Recovery, % | RSD, % | Spiking Levels, μg/kg | Recovery, % | RSD, % | Spiking Levels, μg/kg | Recovery, % | RSD, % | |
| AFB1 | 10 | 101.1 | 5.0 | 2.5 | 106.6 | 6.7 | 5 | 109.4 | 1.6 | 10 | 96.0 | 3.2 |
| 20 | 97.8 | 1.6 | 5 | 100.6 | 0.1 | 10 | 103.3 | 4.1 | 20 | 100.0 | 6.2 | |
| 40 | 108.6 | 0.6 | 10 | 98.7 | 4.0 | 20 | 98.1 | 1.5 | 40 | 108.1 | 0.4 | |
| AFB2 | 2.5 | 94.2 | 4.1 | 0.625 | 105.6 | 7.1 | 1.25 | 109.3 | 2.4 | 2.5 | 92.7 | 2.3 |
| 5 | 90.0 | 0.8 | 1.25 | 100.6 | 0.8 | 2.5 | 102.7 | 4.3 | 5 | 99.3 | 7.0 | |
| 10 | 92.0 | 0.8 | 2.5 | 99.8 | 5.4 | 5 | 97.2 | 1.3 | 10 | 108.9 | 2.4 | |
| AFG1 | 10 | 93.5 | 5.9 | 2.5 | 103.4 | 3.2 | 5 | 106.4 | 0.8 | 10 | 90.3 | 2.0 |
| 20 | 93.5 | 1.4 | 5 | 100.6 | 1.2 | 10 | 102.7 | 1.1 | 20 | 96.6 | 5.5 | |
| 40 | 102.1 | 1.5 | 10 | 99.1 | 5.3 | 20 | 96.3 | 3.0 | 40 | 100.0 | 2.2 | |
| AFG2 | 2.5 | 95.0 | 6.1 | 0.625 | 98.0 | 7.4 | 1.25 | 103.9 | 1.4 | 2.5 | 90.8 | 2.1 |
| 5 | 87.0 | 1.0 | 1.25 | 99.7 | 1.6 | 2.5 | 101.4 | 0.9 | 5 | 92.0 | 4.0 | |
| 10 | 91.9 | 1.5 | 2.5 | 98.4 | 4.8 | 5 | 96.7 | 2.7 | 10 | 85.1 | 3.2 | |
| Matrix | Test Number | Mycotoxin | Detection Value (μg/kg) | Certificate Value (μg/kg) | Range (μg/kg) |
|---|---|---|---|---|---|
| Maize | GBW(E)100386 | AFB1 | 28.5 | 27 | 24–30 |
| FAPAS 04335 | AFB1 | 5.6 | 4.6 | 2.57–6.62 | |
| Husky rice | JTZK-007 | AFB1 | 26.84 | 26 | 22.1–29.9 |
| Peanut oil | JTZK-002 | AFB1 | 15.6 | 15.8 | 13.9–17.7 |
| Rice | JTZK-001 | AFB1 | 10.4 | 9.7 | 8.3–11.1 |
| Sample | Spiking Levels (Low) | Spiking Levels (Medium) | Spiking Levels (High) | Certified Reference Material | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxin | AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 |
| Number of laboratories | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Number of samples | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Number of laboratories retained after eliminating outliers | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Number of accepted results | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Mean value/(μg/kg) | 10.6 | 2.7 | 10.2 | 2.6 | 20.7 | 5.2 | 20.4 | 4.9 | 41.7 | 10.5 | 40.1 | 9.7 | 28.5 | 1.7 |
| Repeatability standard deviation, Sr/(μg/kg) | 0.56 | 0.09 | 0.60 | 0.16 | 0.82 | 0.17 | 0.99 | 0.14 | 1.86 | 0.52 | 2.11 | 0.54 | 1.38 | 0.11 |
| Coefficient of variation of repeatability, Cv,r (%) | 5.3 | 3.5 | 5.9 | 6.4 | 4.0 | 3.2 | 4.9 | 2.9 | 4.5 | 5.0 | 5.3 | 5.6 | 4.9 | 6.3 |
| Repeatability limit r/(μg/kg) | 1.59 | 0.26 | 1.71 | 0.46 | 2.33 | 0.47 | 2.80 | 0.40 | 5.28 | 1.48 | 5.96 | 1.54 | 3.92 | 0.30 |
| Reproducibility standard deviation, SR/(μg/kg) | 0.79 | 0.19 | 0.78 | 0.23 | 1.55 | 0.41 | 1.17 | 0.36 | 2.72 | 0.81 | 2.49 | 0.85 | 2.36 | 0.14 |
| Coefficient of variation of Reproducibility, Cv,R (%) | 7.5 | 7.1 | 7.6 | 8.9 | 7.5 | 7.8 | 5.8 | 7.2 | 6.5 | 7.7 | 6.2 | 8.8 | 8.3 | 8.3 |
| Reproducibility limit R/(μg/kg) | 2.23 | 0.54 | 2.20 | 0.64 | 4.39 | 1.16 | 3.32 | 1.01 | 7.70 | 2.30 | 7.04 | 2.40 | 6.68 | 0.39 |
| HorRat value | 0.67 | 0.52 | 0.68 | 0.64 | 0.74 | 0.63 | 0.57 | 0.58 | 0.72 | 0.69 | 0.68 | 0.78 | 0.86 | 0.56 |
| Recovery (%) | 105.8 | 107.4 | 102.2 | 102.2 | 103.6 | 104.6 | 102.0 | 98.7 | 104.1 | 105.2 | 100.2 | 96.6 | - | - |
| Sample | Spiking Levels (Low) | Spiking Levels (Medium) | Spiking Levels (High) | Reference Material | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxin | AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 |
| Number of laboratories | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Number of samples | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Number of laboratories retained after eliminating outliers | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Number of accepted results | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Mean value/(μg/kg) | 5.0 | 1.3 | 4.6 | 1.2 | 10.4 | 2.6 | 9.9 | 2.5 | 20.1 | 5.0 | 18.9 | 4.8 | 26.8 | 1.4 |
| Repeatability standard deviation, Sr/(μg/kg) | 0.26 | 0.09 | 0.31 | 0.09 | 0.51 | 0.16 | 0.60 | 0.17 | 0.50 | 0.15 | 0.66 | 0.17 | 1.56 | 0.1 |
| Coefficient of variation of repeatability, Cv,r (%) | 5.2 | 6.8 | 6.7 | 7.0 | 5.0 | 6.0 | 6.1 | 6.8 | 2.5 | 2.9 | 3.5 | 3.5 | 5.8 | 7.4 |
| Repeatability limit r/(μg/kg) | 0.75 | 0.25 | 0.88 | 0.24 | 1.45 | 0.45 | 1.70 | 0.48 | 1.41 | 0.41 | 1.88 | 0.47 | 4.43 | 0.3 |
| Reproducibility standard deviation, SR/(μg/kg) | 0.39 | 0.12 | 0.48 | 0.11 | 0.58 | 0.21 | 0.65 | 0.17 | 0.54 | 0.26 | 1.10 | 0.18 | 2.35 | 0.14 |
| Coefficient of variation of Reproducibility, Cv,R (%) | 7.7 | 9.6 | 10.6 | 8.8 | 5.6 | 7.8 | 6.5 | 6.8 | 2.7 | 5.2 | 5.8 | 3.7 | 8.7 | 9.6 |
| Reproducibility limit R/(μg/kg) | 1.10 | 0.35 | 1.37 | 0.30 | 1.65 | 0.59 | 1.84 | 0.48 | 1.52 | 0.74 | 3.10 | 0.50 | 6.64 | 0.38 |
| HorRat value | 0.62 | 0.63 | 0.83 | 0.57 | 0.50 | 0.57 | 0.58 | 0.49 | 0.26 | 0.41 | 0.57 | 0.29 | 0.90 | 0.63 |
| Recovery (%) | 100.5 | 102.5 | 91.9 | 97.5 | 103.7 | 105.7 | 99.4 | 100.0 | 100.5 | 100.7 | 94.4 | 96.3 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Z.; Ye, J.; Zhang, B.; Li, L.; Wu, Y.; Wang, S. An Automated and High-Throughput Immunoaffinity Magnetic Bead-Based Sample Clean-Up Platform for the Determination of Aflatoxins in Grains and Oils Using UPLC-FLD. Toxins 2019, 11, 583. https://doi.org/10.3390/toxins11100583
Xuan Z, Ye J, Zhang B, Li L, Wu Y, Wang S. An Automated and High-Throughput Immunoaffinity Magnetic Bead-Based Sample Clean-Up Platform for the Determination of Aflatoxins in Grains and Oils Using UPLC-FLD. Toxins. 2019; 11(10):583. https://doi.org/10.3390/toxins11100583
Chicago/Turabian StyleXuan, Zhihong, Jin Ye, Bing Zhang, Li Li, Yu Wu, and Songxue Wang. 2019. "An Automated and High-Throughput Immunoaffinity Magnetic Bead-Based Sample Clean-Up Platform for the Determination of Aflatoxins in Grains and Oils Using UPLC-FLD" Toxins 11, no. 10: 583. https://doi.org/10.3390/toxins11100583
APA StyleXuan, Z., Ye, J., Zhang, B., Li, L., Wu, Y., & Wang, S. (2019). An Automated and High-Throughput Immunoaffinity Magnetic Bead-Based Sample Clean-Up Platform for the Determination of Aflatoxins in Grains and Oils Using UPLC-FLD. Toxins, 11(10), 583. https://doi.org/10.3390/toxins11100583