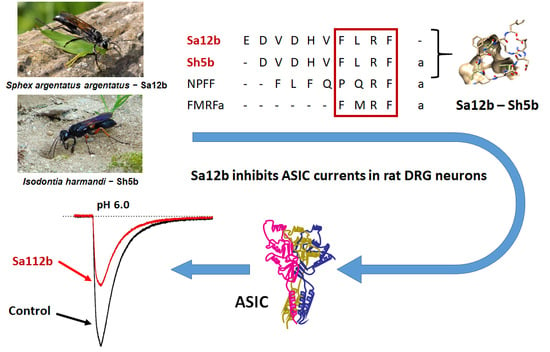
Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons
Abstract
:1. Introduction
2. Results
2.1. ASIC Current in DRG Neurons
2.2. Sa12b Action on ASIC Currents
2.3. pH Activation Versus Sa12b Effect
2.4. Effect of Sh5b
3. Discussion
3.1. Sa12b
3.2. Sh5b
4. Conclusions
5. Material and Methods
5.1. Animals and Cell Culture
5.2. Recording of ASIC Currents in DRG Neurons
5.3. Wasp Peptides
5.4. Peptide Synthesis
5.5. Experimental Design and Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Boscardin, E.; Alijevic, O.; Hummler, E.; Frateschi, S.; Kellenberger, S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19. Br. J. Pharmacol. 2016, 173, 2671–2701. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I. ASIC and ENaC type sodium channels: Conformational states and the structures of the ion selectivity filters. FEBS J. 2017, 284, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I.; Hanukoglu, A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 2016, 579, 95–132. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. International Union of Basic and Clinical Pharmacology. XCI. Structure, Function, and Pharmacology of Acid-Sensing Ion Channels and the Epithelial Na+ Channel. Pharmacol. Rev. 2014, 67, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ramírez, A.; Vega, R.; Soto, E. Acid-Sensing Ion Channels as Potential Therapeutic Targets in Neurodegeneration and Neuroinflammation. Mediat. Inflamm. 2017, 2017, 3728096. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Lingueglia, E. Pharmacology of acid-sensing ion channels—Physiological and therapeutical perspectives. Neuropharmacology 2015, 94, 19–35. [Google Scholar] [CrossRef]
- Cristofori-Armstrong, B.; Rash, L. Acid-sensing ion channel (ASIC) structure and function: Insights from spider, snake and sea anemone venoms. Neuropharmacology 2017, 127, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofori-Armstrong, B.; Saez, N.; Chassagnon, I.; King, G.; Rash, L. The modulation of acid-sensing ion channel 1 by PcTx1 is pH-, subtype- and species-dependent: Importance of interactions at the channel subunit interface and potential for engineering selective analogues. Biochem. Pharmacol. 2019, 163, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, I.; McCarthy, C.; Chin, Y.; Pineda, S.; Keramidas, A.; Mobli, M.; Pham, V.; De Silva, T.M.; Lynch, J.W.; Widdop, R.E.; et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2017, 114, 3750–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbach, J.P.; Grant, P.; Hellemons, A.J.; Degiorgis, J.A.; Li, K.W.; Pant, H.C. Differential expression of the FMRF gene in adult and hatchling stellate ganglia of the squid Loligo pealei. Biol. Open 2014, 3, 50–58. [Google Scholar] [CrossRef]
- Golubovic, A.; Kuhn, A.; Williamson, M.; Kalbacher, H.; Holstein, T.W.; Grimmelikhuijzen, C.J.P.; Gründer, S. A peptide-gated ion channel from the freshwater polyp Hydra. J. Biol. Chem. 2007, 282, 35098–35103. [Google Scholar] [CrossRef] [PubMed]
- Vick, J.S.; Askwith, C.C. ASICs and neuropeptides. Neuropharmacology 2015, 94, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askwith, C.C.; Cheng, C.; Ikuma, M.; Benson, C.; Price, M.P.; Welsh, M.J. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 2000, 26, 133–141. [Google Scholar] [CrossRef]
- Catarsi, S.; Babinski, K.; Séguéla, P. Selective modulation of heteromeric ASIC proton-gated channels by neuropeptide FF. Neuropharmacology 2001, 41, 592–600. [Google Scholar] [CrossRef]
- Deval, E.; Baron, A.; Lingueglia, E.; Mazarguil, H.; Zajac, J.; Lazdunski, M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology 2003, 44, 662–671. [Google Scholar] [CrossRef]
- Xie, J.; Price, M.P.; Wemmie, J.A.; Askwith, C.C.; Welsh, M.J. ASIC3 and ASIC1 Mediate FMRFamide-Related Peptide Enhancement of H+-Gated Currents in Cultured Dorsal Root Ganglion Neurons. J. Neurophysiol. 2003, 89, 2459–2465. [Google Scholar] [CrossRef]
- Sherwood, T.; Askwith, C.C. Endogenous Arginine-Phenylalanine-Amide-related Peptides Alter Steady-state Desensitization of ASIC1a. J. Biol. Chem. 2008, 283, 1818–1830. [Google Scholar] [CrossRef] [Green Version]
- Reimers, C.; Lee, C.H.; Kalbacher, H.; Tian, Y.; Hung, C.H.; Schmidt, A.; Prokop, L.; Kauferstein, S.; Mebs, D.; Chen, C.C.; et al. Identification of a cono-RFamide from the venom of Conus textile that targets ASIC3 and enhances muscle pain. Proc. Natl. Acad. Sci. USA 2017, 114, E3507–E3515. [Google Scholar] [CrossRef]
- Ostrovskaya, O.; Moroz, L.; Krishtal, O. Modulatory action of RFamide-related peptides on acid-sensing ionic channels is pH dependent: The role of arginine. J. Neurochem. 2004, 91, 252–255. [Google Scholar] [CrossRef]
- Lingueglia, E.; Deval, E.; Lazdunski, M. FMRFamide-gated sodium channel and ASIC channels: A new class of ionotropic receptors for FMRFamide and related peptides. Peptides 2006, 27, 1138–1152. [Google Scholar] [CrossRef]
- Chen, X.; Paukert, M.; Kadurin, I.; Pusch, M.; Gründer, S. Strong modulation by RFamide neuropeptides of the ASIC1b/3 heteromer in competition with extracellular calcium. Neuropharmacology 2006, 50, 964–974. [Google Scholar] [CrossRef]
- Frey, E.N.; Pavlovicz, R.E.; Wegman, C.J.; Li, C.; Askwith, C.C. Conformational changes in the lower palm domain of ASIC1a contribute to desensitization and RFamide modulation. PLoS ONE 2013, 8, e71733. [Google Scholar] [CrossRef] [PubMed]
- Bargeton, B.; Iwaszkiewicz, J.; Bonifacio, G.; Roy, S.; Zoete, V.; Kellenberger, S. Mutations in the palm domain disrupt modulation of acid-sensing ion channel 1a currents by neuropeptides. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Peeff, N.M.; Orchard, I.; Lange, A.B. Isolation, sequence, and bioactivity of PDVDHVFLRFamide and ADVGHVFLRFamide peptides from the locust central nervous system. Peptides 1994, 15, 387–392. [Google Scholar] [CrossRef]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide Toxins in Solitary Wasp Venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Petruska, J.C.; Napaporn, J.; Johnson, R.D.; Gu, J.G.; Cooper, B.Y. Subclassified acutely dissociated cells of rat DRG: Histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J. Neurophysiol. 2000, 84, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.; López, O.; Vega, R.; Soto, E. The Aminoglycosides Modulate the Acid-Sensing Ionic Channel Currents in Dorsal Root Ganglion Neurons from the Rat. J. Pharm. Exp. Ther. 2010, 332, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Donier, E.; Rugiero, F.; Jacob, C.; Wood, J.N. Regulation of ASIC activity by ASIC4-new insights into ASIC channel function revealed by a yeast two-hybrid assay. Eur. J. Neurosci. 2008, 28, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.J.; Xie, B.; Wemmie, J.A.; Price, M.P.; Henss, J.M.; Welsh, M.J.; Snyder, P.M. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 2338–2343. [Google Scholar] [CrossRef]
- Xie, J.; Price, M.P.; Berger, A.L.; Welsh, M.J. DRASIC Contributes to pH-Gated Currents in Large Dorsal Root Ganglion Sensory Neurons by Forming Heteromultimeric Channels. J. Neurophysiol. 2002, 87, 2835–2843. [Google Scholar] [CrossRef] [Green Version]
- Lingueglia, E.; de Weille, J.R.; Bassilana, F.; Heurteaux, C.; Sakai, H.; Waldmann, R.; Lazdunski, M. A Modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 1997, 272, 29778–29783. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liu, T.T.; Qu, Z.W.; Qiu, C.Y.; Yang, Z.; Hu, W.P. Gastrodin inhibits the activity of acid-sensing ion channels in rat primary sensory neurons. Eur. J. Pharmacol. 2014, 731, 50–57. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.B.; Hu, L.F.; Yang, Y.P.; Li, J.; Wang, F.; Liu, C.F. ASICs mediate the modulatory effect by paeoniflorin on α-synuclein autophagic degradation. Brain Res. 2011, 1396, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Baron, A.; Rash, L.; Deval, E.; Escoubas, P.; Scarzello, S.; Salinas, M.; Lazdunsky, M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004, 23, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Rash, L.D.; Vila-Farrés, X.; Rosengren, K.J.; Mobli, M.; King, G.F.; Alewood, P.F.; Craik, D.J.; Durek, T. Chemical synthesis, 3D structure, and ASIC binding site of the toxin mambalgin-2. Angew. Chem. Int. Ed. Engl. 2014, 53, 1017–1020. [Google Scholar] [CrossRef]
- Salinas, M.; Besson, T.; Delettre, Q.; Diochot, S.; Boulakirba, S.; Boulakirba, S.; Douguet, D.; Lingueglia, E. Binding site and inhibitory mechanism of the mambalgin-2 pain-relieving peptide on acid- sensing ion channel 1a. J. Biol. Chem. 2014, 289, 13363–13373. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Salceda, E.; Garateix, A.G.; Zaharenko, A.J.; Peigneur, S.; López, O.; Pons, T.; Richardson, M.; Díaz, M.; Hernández, Y.; et al. A novel sea anemone peptide that inhibits acid-sensing ion channels. Peptides 2014, 53, 3–12. [Google Scholar] [CrossRef]
- Chen, X.; Kalbacher, H.; Gründer, S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J. Gen. Physiol. 2006, 127, 267–276. [Google Scholar] [CrossRef]
- Baron, A.; Diochot, S.; Salinas, M.; Deval, E.; Noël, J.; Lingueglia, E. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon 2013, 75, 187–204. [Google Scholar] [CrossRef]
- Baconguis, I.; Gouaux, E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature 2012, 489, 400–405. [Google Scholar] [CrossRef]
- Salceda, E.; Garateix, A.; Soto, E. The sea anemone toxins BgII and BgIII prolong the inactivation time course of the tetrodotoxin-sensitive sodium current in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2002, 303, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Bevan, S.; Hothi, S.; Hughes, G.; James, I.F.; Rang, H.P.; Shah, K.; Walpole, C.S.; Yeats, J.C. Capsazepine: A competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992, 107, 544–552. [Google Scholar] [CrossRef] [PubMed]






| [Sa12b] | n | % Ipeak | % ISS | % Ƭdes | |
|---|---|---|---|---|---|
| Co-application | 30 nM | 5 | 🡹 4 ± 3 | 🡹 39 ± 24 (p = 0.02) | 🡻 4 ± 3 |
| 100 nM | 4 | 🡻 14 ± 5 | 🡻 21 ± 16 | 🡹 5 ± 1 | |
| 300 nM | 6 | 🡻 5 ± 3 | 🡻30 ± 14 | 🡹 3 ± 5 | |
| 1 µM | 5 | 🡹 1 ± 9 | 🡹 15 ± 14 | 🡹 4 ± 4 | |
| 10 µM | 4 | 🡻 13 ± 5 | 🡻 29 ± 19 | 🡻 2 ± 5 | |
| Preincubation Application | 30 nM | 6 | 🡻 0.2 ± 4 | 🡹 19 ± 22 | 🡹 0.03 ± 4 |
| 100 nM | 5 | 🡻 63 ± 4 (p = 0.003) | 🡹 4 ± 21 | 🡹 17 ± 18 | |
| 300 nM | 9 | 🡻 76 ± 5 (p = 0.0002) | 🡹 81 ± 51 | 🡹 28 ± 12 (p = 0.048) | |
| 1 µM | 5 | 🡻 98 ± 1 (p = 0.03) | 🡻 13 ± 27 | ||
| 10 µM | 5 | 🡻 92 ± 7 (p = 0.04) | 🡻 31 ± 20 |
| [Sa12b] | n | % Ipeak | % ISS | % Ƭdes | |
|---|---|---|---|---|---|
| Co-application | 100 nM | 5 | 🡻2 ± 4 | 🡹 85 ± 74 | 🡻 1 ± 2 |
| 3 µM | 12 | 🡻6 ± 3 | 🡹 116 ± 104 | 🡻 7 ± 4 | |
| 10 µM | 9 | 🡹17 ± 15 | 🡹 1 ± 11 | 🡻 10 ± 5 | |
| 30 µM | 6 | 🡻1 ± 6 | 🡹 78 ± 16 | 🡹 9 ± 5 | |
| Preincubation application | 100 nM | 9 | 🡻0.5 ± 6 | 🡹 53 ± 38 | 🡹 13 ± 15 |
| 1 µM | 5 | 🡻9 ± 5 | 🡹 14 ± 41 | 🡹 3 ± 5 | |
| 10 µM | 12 | 🡻11 ± 4 | 🡻 8 ± 15 | 🡻 5 ± 4 |
| Extracellular [mM] | Acid Solution [mM] | Intracellular [mM] | |
|---|---|---|---|
| NaCl | 140 | 140 | 10 |
| KCl | 5.4 | 5.4 | 125 |
| CaCl2 | 1.8 | 1.8 | 0.134 |
| MgCl2 | 1.2 | 1.2 | - |
| HEPES | 10 | - | 5 |
| MES | - | 10 | - |
| D-glucose | 10 | 10 | - |
| EGTA | - | - | 10 |
| ATP- Mg | - | - | 2 |
| GTP-Na | - | - | 1 |
| adjusted to pH 7.4 with NaOH | adjusted to desired pH with NaOH | adjusted to pH 7.2 with KOH |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, C.; Konno, K.; Salceda, E.; Vega, R.; Zaharenko, A.J.; Soto, E. Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons. Toxins 2019, 11, 585. https://doi.org/10.3390/toxins11100585
Hernández C, Konno K, Salceda E, Vega R, Zaharenko AJ, Soto E. Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons. Toxins. 2019; 11(10):585. https://doi.org/10.3390/toxins11100585
Chicago/Turabian StyleHernández, Carmen, Katsuhiro Konno, Emilio Salceda, Rosario Vega, André Junqueira Zaharenko, and Enrique Soto. 2019. "Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons" Toxins 11, no. 10: 585. https://doi.org/10.3390/toxins11100585
APA StyleHernández, C., Konno, K., Salceda, E., Vega, R., Zaharenko, A. J., & Soto, E. (2019). Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons. Toxins, 11(10), 585. https://doi.org/10.3390/toxins11100585