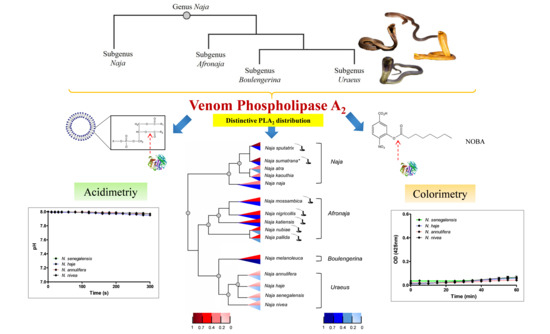
Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus)
Abstract
:1. Introduction
2. Results
2.1. PLA2 Enzymatic Activities (Acidimetric Assay)
2.2. PLA2 Enzymatic Activities (Colorimetric Assay)
2.3. Correlation Between PLA2 Activities and PLA2 Abundances in Cobra Venoms
2.4. Phylogenetics of Cobras in Relation to Venom PLA2 Activities
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Consumables and Reagents
5.2. PLA2 Assay (Acidimetric Method)
5.3. PLA2 Assay (Colorimetric Method)
5.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De, S.S. Physicochemical studies on hemolysin. J. Indian Chem. Soc. 1944, 21, 290. [Google Scholar]
- Tan, C.H.; Tan, N.H. Toxinology of Snake Venoms: The Malaysian Context. In Snake Venoms; Gopalakrishnakone, P., Inagaki, H., Mukherjee, A.K., Rahmy, T.R., Vogel, C.-W., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–37. [Google Scholar]
- Leong, P.K.; Fung, S.Y.; Tan, C.H.; Sim, S.M.; Tan, N.H. Immunological cross-reactivity and neutralization of the principal toxins of Naja sumatrana and related cobra venoms by a Thai polyvalent antivenom (Neuro Polyvalent Snake Antivenom). Acta Tropica 2015, 149, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.M.F.; Tan, C.H.; Tan, K.Y.; Quraishi, N.A.; Tan, N.H. Venom proteome of Bungarus sindanus (Sind krait) from Pakistan and in vivo cross-neutralization of toxicity using an Indian polyvalent antivenom. J. Proteomics 2019, 193, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Liew, J.L.; Tan, N.H.; Quah, E.S.H.; Ismail, A.K.; Tan, C.H. Unlocking the secrets of banded coral snake (Calliophis intestinalis, Malaysia): A venom with proteome novelty, low toxicity and distinct antigenicity. J. Proteomics 2019, 192, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Fung, S.Y.; Yap, M.K.; Leong, P.K.; Liew, J.L.; Tan, N.H. Unveiling the elusive and exotic: Venomics of the Malayan blue coral snake (Calliophis bivirgata flaviceps). J. Proteomics 2016, 132, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-H. Snake Venom Phospholipase A2: Evolution and Diversity. In Venom Genomics and Proteomics: Venom Genomics and Proteomics; Gopalakrishnakone, P., Calvete, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–13. [Google Scholar]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.W.; Harris, J.B. Nerve Terminal Damage by β-Bungarotoxin: Its Clinical Significance. Am. J. Pathol. 1999, 154, 447–455. [Google Scholar] [CrossRef]
- Oh, A.M.F.; Tan, C.H.; Ariaranee, G.C.; Quraishi, N.; Tan, N.H. Venomics of Bungarus caeruleus (Indian krait): Comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. J. Proteomics 2017, 164, 1–18. [Google Scholar] [CrossRef]
- Ramalingam, K.; Karthigayan, S.; Muralimanoharan, S.; Vijayalakshmi, S.; Thangavel, B. Histopathological changes induced in mice after inramuscular and intraperitoneal injections of venom from spine-bellied sea snake, Lapemis curtus (Shaw, 1802). J. Pharmacol. Toxicol. 2007, 2, 307–318. [Google Scholar]
- Brook, G.A.; Torres, L.F.; Gopalakrishnakone, P.; Duchen, L.W. Effects of phospholipase of Enhydrina schistosa venom on nerve, motor end-plate and muscle of the mouse. Q. J. Exp. Physiol. 1987, 72, 571–591. [Google Scholar] [CrossRef]
- Huang, M.Z.; Wang, Q.C.; Liu, G.F. Effects of an acidic phospholipase A2 purified from Ophiophagus hannah (king cobra) venom on rat heart. Toxicon 1993, 31, 627–635. [Google Scholar] [CrossRef]
- Tan, N.H.; Wong, K.Y.; Tan, C.H. Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization. J. Proteomics 2017, 157, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.-H. Isolation and preliminary characterisation of two toxic phospholipases A2 from the venom of the Malayan cobra (Naja naja sputatrix). BBA-Gen. Subj. 1982, 719, 599–605. [Google Scholar] [CrossRef]
- Choudhury, M.; McCleary, R.J.R.; Kesherwani, M.; Kini, R.M.; Velmurugan, D. Comparison of proteomic profiles of the venoms of two of the ‘Big Four’ snakes of India, the Indian cobra (Naja naja) and the common krait (Bungarus caeruleus), and analyses of their toxins. Toxicon 2017, 135, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteomics 2017, 156, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.D.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J. Proteomics 2016, 132, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J. Proteomics 2018, 175, 156–173. [Google Scholar] [CrossRef]
- Liu, C.-C.; You, C.-H.; Wang, P.-J.; Yu, J.-S.; Huang, G.-J.; Liu, C.-H.; Hsieh, W.-C.; Lin, C.-C. Analysis of the efficacy of Taiwanese freeze-dried neurotoxic antivenom against Naja kaouthia, Naja siamensis and Ophiophagus hannah through proteomics and animal model approaches. PLOS Negl. Trop. Dis. 2017, 11, e0006138. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteomics 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteomics 2015, 120, 105–125. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutierrez, J.M.; Lohse, B.; Rasmussen, A.R.; Fernandez, J.; Milbo, C.; Lomonte, B. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 2015, 99, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhao, H.Y.; Yin, Y.; Shen, S.S.; Shan, L.L.; Chen, C.X.; Zhang, Y.X.; Gao, J.F.; Ji, X. Combined venomics, antivenomics and venom gland transcriptome analysis of the monocoled cobra (Naja kaouthia) from China. J. Proteomics 2017, 159, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutierrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteomics 2017, 150, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Sanz, L.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the Pan-African EchiTAb-Plus-ICP Antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Hus, K.; Buczkowicz, J.; Petrilla, V.; Petrillová, M.; Łyskowski, A.; Legáth, J.; Bocian, A. First Look at the venom of Naja ashei. Molecules 2018, 23, 609. [Google Scholar] [CrossRef]
- Malih, I.; Ahmad rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic analysis of Moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteomics 2014, 96, 240–252. [Google Scholar] [CrossRef]
- Mebs, D. A comparative study of enzyme activities in snake venoms. Int. J. Biochem. 1970, 1, 335–342. [Google Scholar] [CrossRef]
- Tan, N.-H.; Tan, C.-S. A comparative study of cobra (Naja) venom enzymes. Comp. Biochem. Physiol. B Comp. Biochem. 1988, 90, 745–750. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 15 January 2019).
- Wallach, V.; Wüster, W.; Broadley, D.G. In praise of subgenera: taxonomic status of cobras of the genus Naja Laurenti (Serpentes: Elapidae). Zootaxa 2009, 2236, 26–36. [Google Scholar]
- Wüster, W. Taxonomic changes and toxinology: Systematic revisions of the Asiatic cobras (Naja naja species complex). Toxicon 1996, 34, 399–406. [Google Scholar] [CrossRef]
- Yap, M.K.; Fung, S.Y.; Tan, K.Y.; Tan, N.H. Proteomic characterization of venom of the medically important Southeast Asian Naja sumatrana (Equatorial spitting cobra). Acta Trop. 2014, 133, 15–25. [Google Scholar] [CrossRef]
- Holzer, M.; Mackessy, S.P. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Bacha, A.B.; Alonazi, M.A.; Elshikh, M.S.; Karray, A. A novel bactericidal homodimeric PLA2 group-I from Walterinnesia aegyptia venom. Int. J. Biol. Macromol. 2018, 117, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.J.; Washburn, W.N.; Deems, R.A.; Dennis, E.A. Assay strategies and methods for phospholipases. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1991; Volume 197, pp. 3–23. [Google Scholar]
- Cho, W.; Kezdy, F.J. Chromogenic substrates and assay of phospholipases A2. Methods Enzymol. 1991, 197, 75–79. [Google Scholar] [PubMed]
- Gowda, T.V.; Middlebrook, J.L. Effects of myonecrotic snake venom phospholipase A2 toxins on cultured muscle cells. Toxicon 1993, 31, 1267–1278. [Google Scholar] [CrossRef]
- Mebs, D. Myotoxic activity of phospholipases A2 isolated from cobra venoms: Neutralization by polyvalent antivenoms. Toxicon 1986, 24, 1001–1008. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Zdenek, C.N.; Op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic cobras: Clinical implications of strong anticoagulant actions of African spitting Naja Venoms that are not neutralised by antivenom but are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.G.; Gebi, U.I.; Onyemelukwe, G.C. Snake bite in Nigeria. Afr. J. Med. Med. Sci. 2001, 30, 171–178. [Google Scholar]
- WHO. Guidelines for the Prevention and Clinical Management of Snakebite in Africa; WHO Regional Office for Africa: Brazzaville, Congo, 2010. [Google Scholar]
- Tan, N.H.; Armugam, A. In vivo interactions between neurotoxin, cardiotoxin and phospholipases A2 isolated from Malayan cobra (Naja naja sputatrix) venom. Toxicon 1990, 28, 1193–1198. [Google Scholar] [CrossRef]
- Bougis, P.E.; Marchot, P.; Rochat, H. In vivo synergy of cardiotoxin and phospholipase A2 from the elapid snake Naja mossambica mossambica. Toxicon 1987, 25, 427–431. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the principal toxins from the venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into toxin-specific neutralization by two different antivenoms. Toxins 2016, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, C.H.; Tan, N.H. Venom and purified toxins of the spectacled cobra (Naja naja) from Pakistan: Insights into toxicity and antivenom neutralization. Am. J. Trop. Med. Hyg. 2016, 94, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Kalita, B.; Thakur, R. Two Acidic, Anticoagulant PLA2 isoenzymes purified from the venom of monocled cobra Naja kaouthia exhibit different potency to inhibit thrombin and factor xa via phospholipids independent, non-enzymatic mechanism. PLoS ONE 2014, 9, e101334. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Zhou, X.; Kini, R.M. Snake venom phospholipase A2 enzymes; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Lynch, V.J. Inventing an arsenal: Adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol. Biol. 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Ratanabanangkoon, K.; Tan, K.Y.; Eursakun, S.; Tan, C.H.; Simsiriwong, P.; Pamornsakda, T.; Wiriyarat, W.; Klinpayom, C.; Tan, N.H. A simple and novel strategy for the production of a pan-specific antiserum against Elapid snakes of Asia. PLoS Negl. Trop. Dis. 2016, 10, e0004565. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Liew, J.L.; Tan, K.Y.; Tan, N.H. Assessing SABU (Serum Anti Bisa Ular), the sole Indonesian antivenom: A proteomic analysis and neutralization efficacy study. Sci. Rep. 2016, 6, 37299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wüster, W.; Thorpe, R.S. Dentitional phenomena in cobras revisited: Spitting and fang structure in the Asiatic species of Naja (Serpentes: Elapidae). Herpetologica 1992, 48, 424–434. [Google Scholar]
- Panagides, N.; Jackson, T.N.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the cobra got its flesh-eating venom: Cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef]
- Chong, H.P.; Tan, K.Y.; Tan, N.H.; Tan, C.H. Exploring the diversity and novelty of toxin genes in Naja sumatrana, the Equatorial spitting cobra from Malaysia through de novo venom-gland transcriptomics. Toxins 2019, 11, 104. [Google Scholar] [CrossRef]
- Tan, N.-H.; Tan, C.-S. Acidimetric assay for phospholipase A using egg yolk suspension as substrate. Anal. Biochem. 1988, 170, 282–288. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; Damico, D.C.S.; Ponce-Soto, L.A.; Baldasso, P.A.; Da Silva, S.L.; Souza, G.H.M.F.; Eberlin, M.N.; Marangoni, S. Biological and biochemical characterization of new basic phospholipase A2 BmTX-I isolated from Bothrops moojeni snake venom. Toxicon 2008, 51, 1509–1519. [Google Scholar] [CrossRef] [PubMed]




| Subgenus | Cobra Venom | Relative PLA2 Abundance (% Total Venom Proteins) | PLA2 Activity of Venom (nmol/min/mg Venom Proteins) | PLA2 Specific Activity (nmol/min/mg PLA2 Protein) |
|---|---|---|---|---|
| Naja | N. naja | 14.2 | 39.57 | 277.88 |
| N. kaouthia | 12.2 | 42.26 | 346.38 | |
| N. sputatrix | 31.2 | 109.70 | 351.13 | |
| N. atra | 14.4 | 33.21 | 230.12 | |
| N. sumatrana | 32.3 | 82.11 | 254.21 | |
| Afronaja | N. nigricollis | 21.9 | 45.85 | 209.36 |
| N. pallida | 30.1 | 52.78 | 175.36 | |
| N. nubiae | 26.4 | 53.82 | 203.84 | |
| N. mossambica | 27.1 | 45.35 | 167.36 | |
| N. katiensis | 29.0 | 45.15 | 155.69 | |
| Boulengerina | N. melanoleuca | 12.9 | 48.03 | 372.33 |
| Uraeus | N. haje | 4.0 | 10.87 | 271.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.H.; Wong, K.Y.; Tan, N.H.; Ng, T.S.; Tan, K.Y. Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins 2019, 11, 116. https://doi.org/10.3390/toxins11020116
Tan CH, Wong KY, Tan NH, Ng TS, Tan KY. Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins. 2019; 11(2):116. https://doi.org/10.3390/toxins11020116
Chicago/Turabian StyleTan, Choo Hock, Kin Ying Wong, Nget Hong Tan, Tzu Shan Ng, and Kae Yi Tan. 2019. "Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus)" Toxins 11, no. 2: 116. https://doi.org/10.3390/toxins11020116
APA StyleTan, C. H., Wong, K. Y., Tan, N. H., Ng, T. S., & Tan, K. Y. (2019). Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins, 11(2), 116. https://doi.org/10.3390/toxins11020116