Diverse Components of Resistance to Fusarium verticillioides Infection and Fumonisin Contamination in Four Maize Recombinant Inbred Families
Abstract
:1. Introduction
2. Results
2.1. Symptomatology Varies among Families
2.2. Relationships among External Symptomatology, Kernel Bulk Density, and Toxin Load Vary among Families
2.3. Fusarium Ear Rot Severity, Fumonisin Contamination, and Kernel Bulk Density are Correlated
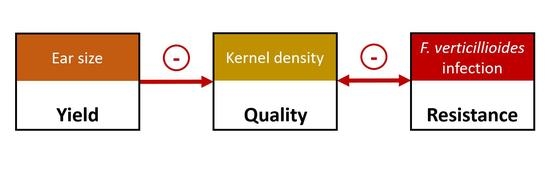
2.4. Innate Ear Morphology is a Component of Resistance to FVI and FUM Contamination
2.5. Genetic and Environmental Variation on Disease Severity Differ among Families
2.6. Genetic Architecture Differs between Ear Morphological and Resistance Traits
2.7. Most QTL for Resistance to F. verticillioides are Trait-Specific
2.8. Allele Effects at FVI-Specific Loci Reflect Trait Relationships
2.9. Characteristics of Loci Underlying Specific and Ear-Mediated Resistance to F. verticillioides
3. Discussion
4. Materials and Methods
4.1. Field Design and Inoculation
4.2. Disease Phenotyping
4.3. Mixed Models and Heritability Estimation
4.4. Trait Correlation Analyses
4.5. Comparison of Disease Severity among Families
4.6. QTL Mapping, Colocalization, and Allele Effects
4.7. Characterization of Resistance QTL
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by Fusarium Species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bosley, D.B.; Bradley, C.A.; Broders, K.D.; Byamukama, E.; Chilvers, M.I.; et al. Corn Yield Loss Estimates Due to Diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Prog. 2016, 17, 211–222. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and Human Disease: A Largely Ignored Global Health Issue. Carcinogenesis 2009, 31, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins Disrupt Sphingolipid Metabolism, Folate Transport, and Neural Tube Development in Embryo Culture and in Vivo: A Potential Risk Factor for Human Neural Tube Defects among Populations Consuming Fumonisin-Contaminated Maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Alfred, H.; Rothman, K.J.; Hendricks, K.A. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas-Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kimanya, M.E.; De Meulenaer, B.; Roberfroid, D.; Lachat, C. Fumonisin Exposure through Maize in Complementary Foods is Inversely Associated with Linear Growth of Infants in Tanzania. Mol. Nutr. Food Res. 2010, 54, 1659–1667. [Google Scholar] [CrossRef]
- Kellerman, T.; Marasas, W.; Thiel, P.; Gelderblom, W.C.A.; Cawood, M.; Coetzer, J. Leukoencephalomalacia in Two Horses Induced by Oral Dosing of Fumonisin B1. Onderstepoort J. Vet. Res. 1990, 57, 269–275. [Google Scholar]
- Colvin, B.M.; Harrison, L.R. Fumonisin-Induced Pulmonary Edema and Hydrothorax in Swine. Mycopathologia 1992, 117, 79–82. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Extent and Drivers of Mycotoxin Contamination: Inferences from a Survey of Kenyan Maize Mills. Phytopathology 2014, 104, 1221–1231. [Google Scholar] [CrossRef]
- Bankole, S.A.; Mabekoje, O.O. Occurrence of Aflatoxins and Fumonisins in Preharvest Maize from South-Western Nigeria. Food Addit. Contam. 2004, 21, 251–255. [Google Scholar] [CrossRef]
- Fandohan, P.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Natural Occurrence of Fusarium and Subsequent Fumonisin Contamination in Preharvest and Stored Maize in Benin, West Africa. Int. J. Food Microbiol. 2005, 99, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Arino, A.; Herrera, M.; Juan, T.; Estopanan, G.; Carraminana, J.J.; Rota, C.; Herrera, A. Influence of Agricultural Practices on the Contamination of Maize by Fumonisin Mycotoxins. J. Food Prot. 2009, 72, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Reyneri, A.; Vanara, F. Influence of Nitrogen Fertilization on Mycotoxin Contamination of Maize Kernels. Crop Prot. 2008, 27, 222–230. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular Basis of Resistance to Fusarium Ear Rot in Maize. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Bandyopadhyay, R.; Leslie, J.F.; Ekpo, E.J.A. Effect of Sorting on Incidence and Occurrence of Fumonisins and Fusarium verticillioides on Maize from Nigeria. J. Food Prot. 2006, 69, 2019–2023. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Falade, T.D.O.; Mutuma, M.; Mutiga, S.K.; Harvey, J.J.W.; Fox, G.; Pearson, T.C.; Muthomi, J.W.; Nelson, R.J. Multi-Spectral Kernel Sorting to Reduce Aflatoxins and Fumonisins in Kenyan Maize. Food Control 2017, 78, 203–214. [Google Scholar] [CrossRef]
- Dombrink-Kurtzman, M.A.; Dvorak, T.J.; Barron, M.E.; Rooney, L.W. Effect of Nixtamalization (Alkaline Cooking) on Fumonisin-Contaminated Corn for Production of Masa and Tortillas. J. Agric. Food Chem. 2000, 48, 5781–5786. [Google Scholar] [CrossRef]
- Humpf, H.-U.; Voss, K.A. Effects of Thermal Food Processing on the Chemical Structure and Toxicity of Fumonisin Mycotoxins. Mol. Nutr. Food Res. 2004, 48, 255–269. [Google Scholar] [CrossRef]
- Robertson-Hoyt, L.A.; Jines, M.P.; Balint-Kurti, P.J.; Kleinschmidt, C.E.; White, D.G.; Payne, G.A.; Maragos, C.M.; Molna, T.L.; Holland, J.B. QTL Mapping for Fusarium Ear Rot and Fumonisin Contamination Resistance in Two Maize Populations. Crop Sci. 2006, 46, 1734–1743. [Google Scholar] [CrossRef]
- Zila, C.T.; Ogut, F.; Romay, M.C.; Gardner, C.A.; Buckler, E.S.; Holland, J.B. Genome-Wide Association Study of Fusarium Ear Rot Disease in the U.S.A. Maize Inbred Line Collection. BMC Plant Biol. 2014, 14, 372. [Google Scholar] [CrossRef]
- Zila, C.T.; Samayoa, L.F.; Santiago, R.; Butrón, A.; Holland, J.B. A Genome-Wide Association Study Reveals Genes Associated with Fusarium Ear Rot Resistance in a Maize Core Diversity Panel. G3 2013, 3, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.A.; Balint-Kurti, P.J.; Wisser, R.J.; Pratt, R.C.; Nelson, R.J. Shades of Gray: The World of Quantitative Disease Resistance. Trends Plant Sci. 2009, 14, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Giorni, P.; Cirlini, M.; Reverberi, M.; Gregori, R.; Ludovici, M.; Camera, E.; Fanelli, C.; Battilani, P.; Scala, V. Maize Lipids Play a Pivotal Role in the Fumonisin Accumulation. World Mycotoxin J. 2015, 8, 87–97. [Google Scholar] [CrossRef]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional Genomic Analysis of Constitutive and Inducible Defense Responses to Fusarium verticillioides Infection in Maize Genotypes with Contrasting Ear Rot Resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Gao, J.; Wu, Y.; Xia, Z.; Zhang, H.; Henry, R. The Mechanisms of Maize Resistance to Fusarium verticillioides by Comprehensive Analysis of RNA-Seq Data. Front. Plant Sci. 2016, 7, 1654. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.A.; Fauguel, C.M.; Vattuone, M.A.; Presello, D.A.; Catalán, C.A.N. Phenylpropanoids from Maize Pericarp: Resistance Factors to Kernel Infection and Fumonisin Accumulation by Fusarium verticillioides. Eur. J. Plant Pathol. 2013, 135, 105–113. [Google Scholar] [CrossRef]
- Burr, S.; Fry, S. Feruloylated Arabinoxylans Are Oxidatively Cross-Linked by Extracellular Maize Peroxidase but Not by Horseradish Peroxidase. Mol. Plant 2009, 2, 883–892. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A. Effect of Maize Hybrid Maturity and Grain Hardness on Fumonisin and Zearalenone Contamination. Ital. J. Agron. 2008, 2, 107–117. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Vattuone, M.A.; Presello, D.A.; Fauguel, C.M. The Pericarp and Its Surface Wax Layer in Maize Kernels as Resistance Factors to Fumonisin Accumulation by Fusarium verticillioides. Crop Prot. 2009, 28, 196–200. [Google Scholar] [CrossRef]
- Bluhm, B.H.; Woloshuk, C.P. Amylopectin Induces Fumonisin B1 Production by Fusarium verticillioides during Colonization of Maize Kernels. Mol. Plant-Microbe Interact. 2005, 18, 1333–1339. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Morales, L.; Angwenyi, S.; Wainaina, J.; Harvey, J.; Das, B.; Nelson, R.J. Association between Agronomic Traits and Aflatoxin Accumulation in Diverse Maize Lines Grown under Two Soil Nitrogen Levels in Eastern Kenya. Field Crop. Res. 2017, 205, 124–134. [Google Scholar] [CrossRef]
- Shetty, P.H.; Bhat, R.V. A Physical Method for Segregation of Fumonisin-Contaminated Maize. Food Chem. 1999, 66, 371–374. [Google Scholar] [CrossRef]
- Morales, L.; Marino, T.P.; Wenndt, A.J.; Fouts, J.Q.; Holland, J.B.; Nelson, R.J. Dissecting Symptomatology and Fumonisin Contamination Produced by Fusarium verticillioides in Maize Ears. Phytopathology 2018, 108, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.P. Genomic Prediction and QTL Validation for Resistance to Fusarium Ear Rot and Fumonisin in Maize. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, October 2018. [Google Scholar]
- Horne, D.W.; Eller, M.S.; Holland, J.B. Responses to Recurrent Index Selection for Reduced Fusarium Ear Rot and Lodging and for Increased Yield in Maize. Crop Sci. 2016, 56, 85–94. [Google Scholar] [CrossRef]
- Jansen, C.; Zhang, Y.; Liu, H.; Gonzalez-Portilla, P.J.; Lauter, N.; Kumar, B.; Trucillo-Silva, I.; Martin, J.P.S.; Lee, M.; Simcox, K.; et al. Genetic and Agronomic Assessment of Cob Traits in Corn under Low and Normal Nitrogen Management Conditions. Theor. Appl. Genet. 2015, 128, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.W.; Munkvold, G.P. Effects of Planting Date and Environmental Factors on Fusarium Ear Rot Symptoms and Fumonisin B1 Accumulation in Maize Grown in Six North American Locations. Plant Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Associations of Planting Date, Drought Stress, and Insects with Fusarium Ear Rot and Fumonisin B1 Contamination in California Maize. Food Addit. Contam. 2010, 27, 591–607. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D.; Lu, M.; Claflin, L.W. Distribution of Fumonisins in Maize Ears Infected with Strains of Fusarium moniliforme That Differ in Fumonisin Production. Plant Dis. 1998, 82, 953–958. [Google Scholar] [CrossRef]
- Yu, J.; Holland, J.B.; McMullen, M.D.; Buckler, E.S. Genetic Design and Statistical Power of Nested Association Mapping in Maize. Genetics 2008, 178, 539–551. [Google Scholar] [CrossRef]
- Brown, P.J.; Upadyayula, N.; Mahone, G.S.; Tian, F.; Bradbury, P.J.; Myles, S.; Holland, J.B.; Flint-Garcia, S.; McMullen, M.D.; Buckler, E.S.; et al. Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize. PLoS Genet. 2011, 7, e1002383. [Google Scholar] [CrossRef]
- Zhao, W. Panzea: A Database and Resource for Molecular and Functional Diversity in the Maize Genome. Nucleic Acids Res. 2006, 34, D752–D757. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.A.; Kleinschmidt, C.E.; White, D.G.; Payne, G.A.; Maragos, C.M.; Holland, J.B. Heritabilities and Correlations of Fusarium Ear Rot Resistance and Fumonisin Contamination Resistance in Two Maize Populations. Crop Sci. 2006, 46, 353–361. [Google Scholar] [CrossRef]
- Hung, H.Y.; Holland, J.B. Diallel Analysis of Resistance to Fusarium Ear Rot and Fumonisin Contamination in Maize. Crop Sci. 2012, 52, 2173–2181. [Google Scholar] [CrossRef]
- Zummo, N.; Scott, G.E. Cob and Kernel Infection by Aspergillus flavus and Fusarium moniliforme in Inoculated, Field-Grown Maize Ears. Plant Dis. 1990, 74, 627–631. [Google Scholar] [CrossRef]
- Presello, D.A.; Botta, G.; Iglesias, J.; Eyhérabide, G.H. Effect of Disease Severity on Yield and Grain Fumonisin Concentration of Maize Hybrids Inoculated with Fusarium verticillioides. Crop Prot. 2008, 27, 572–576. [Google Scholar] [CrossRef]
- Presello, D.A.; Iglesias, J.; Botta, G.; Eyherabide, G.H. Severity of Fusarium Ear Rot and Concentration of Fumonisin in Grain of Argentinian Maize Hybrids. Crop Prot. 2007, 26, 852–855. [Google Scholar] [CrossRef]
- Robertson-Hoyt, L.A.; Betrán, J.; Payne, G.A.; White, D.G.; Isakeit, T.; Maragos, C.M.; Molnár, T.L.; Holland, J.B. Relationships Among Resistances to Fusarium and Aspergillus Ear Rots and Contamination by Fumonisin and Aflatoxin in Maize. Phytopathology 2007, 97, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Mideros, S.X.; Warburton, M.L.; Jamann, T.M.; Windham, G.L.; Williams, W.P.; Nelson, R.J. Quantitative Trait Loci Influencing Mycotoxin Contamination of Maize: Analysis by Linkage Mapping, Characterization of Near-Isogenic Lines, and Meta-Analysis. Crop Sci. 2014, 54, 127. [Google Scholar] [CrossRef]
- Fox, G.; Manley, M. Hardness Methods for Testing Maize Kernels. J. Agric. Food Chem. 2009, 57, 5647–5657. [Google Scholar] [CrossRef]
- Kandianis, C.B.; Michenfelder, A.S.; Simmons, S.J.; Grusak, M.A.; Stapleton, A.E. Abiotic Stress Growth Conditions Induce Different Responses in Kernel Iron Concentration across Genotypically Distinct Maize Inbred Varieties. Front. Plant Sci. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Rodgers-Melnick, E.; Bradbury, P.J.; Elshire, R.J.; Glaubitz, J.C.; Acharya, C.B.; Mitchell, S.E.; Li, C.; Li, Y.; Buckler, E.S. Recombination in Diverse Maize Is Stable, Predictable, and Associated with Genetic Load. Proc. Natl. Acad. Sci. USA 2015, 112, 201413864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shrestha, R.; Ding, J.; Zheng, H.; Mu, C.; Wu, J.; Mahuku, G. Genome-Wide Association Study and QTL Mapping Reveal Genomic Loci Associated with Fusarium Ear Rot Resistance in Tropical Maize Germplasm. G3 2016, 8651, g3-116. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.S.; Payne, G.A.; Holland, J.B. Selection for Reduced Fusarium Ear Rot and Fumonisin Content in Advanced Backcross Maize Lines and Their Topcross Hybrids. Crop Sci. 2010, 50, 2249–2260. [Google Scholar] [CrossRef]
- Ding, J.-Q.; Wang, X.-M.; Chander, S.; Yan, J.-B.; Li, J.-S. QTL Mapping of Resistance to Fusarium Ear Rot Using a RIL Population in Maize. Mol. Breed. 2008, 22, 395–403. [Google Scholar] [CrossRef]
- Xiang, K.; Zhang, Z.M.; Reid, L.M.; Zhu, X.Y.; Yuan, G.S.; Pan, G.T. A Meta-Analysis of QTL Associated with Ear Rot Resistance in Maize. Maydica 2010, 55, 281–290. [Google Scholar]
- Maschietto, V.; Colombi, C.; Pirona, R.; Pea, G.; Strozzi, F.; Marocco, A.; Rossini, L.; Lanubile, A. QTL Mapping and Candidate Genes for Resistance to Fusarium Ear Rot and Fumonisin Contamination in Maize. BMC Plant Biol. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Giomi, G.M.; Kreff, E.D.; Iglesias, J.; Fauguel, C.M.; Fernandez, M.; Oviedo, M.S.; Presello, D.A. Quantitative Trait Loci for Fusarium and Gibberella Ear Rot Resistance in Argentinian Maize Germplasm. Euphytica 2016, 211, 287–294. [Google Scholar] [CrossRef]
- Perez-Brito, D.; Jeffers, D.; Gonzalez-de-Leon, D.; Khairallah, M.; Cortes-Cruz, M.; Velazquez-Cardelas, G.; Azpiroz-Rivero, S.; Srinivasan, G. QTL Mapping of Fusarium moniliforme Ear Rot Resistance in High Land Maize, Mexico. Agrociencia 2001, 35, 181–196. [Google Scholar]
- Clements, M.J.; Kleinschmidt, C.E.; Maragos, C.M.; Pataky, J.K.; White, D.G. Evaluation of Inoculation Techniques for Fusarium Ear Rot and Fumonisin Contamination of Corn. Plant Dis. 2003, 87, 147–153. [Google Scholar] [CrossRef]
- Gustin, J.L.; Jackson, S.; Williams, C.; Patel, A.; Armstrong, P.; Peter, G.F.; Settles, A.M. Analysis of Maize (Zea mays) Kernel Density and Volume Using Microcomputed Tomography and Single-Kernel Near-Infrared Spectroscopy. J. Agric. Food Chem. 2013, 61, 10872–10880. [Google Scholar] [CrossRef]
- Shelby, R.A.; White, D.G.; Bauske, E.M. Differential Fumonisin Production in Maize Hybrids. Plant Dis. 1994, 78, 582–584. [Google Scholar] [CrossRef]
- Buckler, E.S.; Holland, J.B.; Bradbury, P.J.; Acharya, C.B.; Brown, P.J.; Browne, C.; Ersoz, E.; Flint-Garcia, S.; Garcia, A.; Glaubitz, J.C.; et al. The Genetic Architecture of Maize Flowering Time. Science 2009, 325, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Môro, F.V.; Môro, J.R.; da Silva, H.P.; Panizzi, R.D.C. Relação Entre Características Morfológicas Da Cariopse e Fusariose Em Milho. Pesqui. Agropecu. Bras. 2003, 38, 27–33. [Google Scholar] [CrossRef]
- Duncan, K.E.; Howard, R.J. Biology of Maize Kernel Infection by Fusarium verticillioides. Mol. Plant-Microbe Interact. 2010, 23, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.; Lipka, A.; Magallanes-Lundback, M.; Tiede, T.; Diepenbrock, C.; Kandianis, C.B.; Kim, E.; Cepela, J.; Mateos-Hernandez, M.; Buell, C.; et al. A Foundation for Provitamin A Biofortification of Maize: Genome-Wide Association and Genomic Prediction Models of Carotenoid Levels. Genetics 2014, 198, 1699–1716. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.B.; Nyquist, W.E.; Cervantes-Martinez, C.T. Estimating and Interpreting Heritability for Plant Breeding. Plant Breed. Rev. 2003, 22, 9–112. [Google Scholar]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Olukolu, B.A.; Wang, G.F.; Vontimitta, V.; Venkata, B.P.; Marla, S.; Ji, J.; Gachomo, E.; Chu, K.; Negeri, A.; Benson, J.; et al. A Genome-Wide Association Study of the Maize Hypersensitive Defense Response Identifies Genes That Cluster in Related Pathways. PLoS Genet. 2014, 10, e1004562. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Romay, M.C.; Gore, M.A.; Flint-Garcia, S.A.; Zhang, Z.; Millard, M.J.; Gardner, C.A.C.; McMullen, M.D.; Holland, J.B.; Bradbury, P.J.; et al. The Genetic Architecture of Maize Height. Genetics 2014, 196, 1337–1356. [Google Scholar] [CrossRef]
- Andorf, C.M.; Cannon, E.K.; Portwood, J.L.; Gardiner, J.M.; Harper, L.C.; Schaeffer, M.L.; Braun, B.L.; Campbell, D.A.; Vinnakota, A.G.; Sribalusu, V.V.; et al. MaizeGDB Update: New Tools, Data and Interface for the Maize Model Organism Database. Nucleic Acids Res. 2016, 44, D1195–D1201. [Google Scholar] [CrossRef] [PubMed]


| Family | RILs (N/env) | BDENinoc *** (g mL−1) | FER*** (%) | FUM*** (ppm) | FUM:FER *** (ppm %−1) |
|---|---|---|---|---|---|
| B73 × CML333 | 186 | 0.737 ± 0.003 A | 43.04 ± 1.27 A | 24.24 ± 5.32 B | 0.51 ± 0.10 C |
| B73 × CML52 | 177 | 0.720 ± 0.004 B | 34.41 ± 1.05 B | 11.77 ± 2.39 B | 0.60 ± 0.08 B |
| B73 × CML69 | 186 | 0.712 ± 0.003 B | 40.04 ± 1.23 A | 31.28 ± 5.05 A | 1.00 ± 0.13 AB |
| B73 × NC358 | 179 | 0.683 ± 0.004 C | 43.27 ± 1.20 A | 32.62 ± 4.21 A | 1.19 ± 0.23 A |
| Parent | Plots (N/env) | BDENinoc *** (g mL−1) | FER*** (%) | FUM*** (ppm) | FUM:FER (ppm %−1) |
| B73 | 39 | 0.650 ± 0.005 C | 47.83 ± 2.26 A | 72.93 ± 14.41 A | 2.36 ± 0.64 A |
| CML333 | 11 | 0.770 ± 0.008 A | 26.65 ± 3.37 B | 7.09 ± 2.82 B | 0.47 ± 0.18 B |
| CML52 | 11 | 0.714 ± 0.020 B | 13.39 ± 3.17 C | 3.18 ± 0.78 B | 0.76 ± 0.26 AB |
| CML69 | 11 | 0.745 ± 0.004 AB | 25.42 ± 5.89 B | 6.69 ± 2.08 B | 0.48 ± 0.20 AB |
| NC358 | 11 | 0.766 ± 0.007 A | 26.18 ± 3.67 B | 16.14 ± 7.36 B | 0.63 ± 0.25 AB |
| Family | Symptom type | BDENinoc (g mL−1) | FER (%) | FUM (ppm) | FUM:FER (ppm %−1) |
|---|---|---|---|---|---|
| Combined | Asym. | 0.693 ± 0.003 A | 19.58 ± 0.44 E | 10.79 ± 1.69 A | 0.96 ± 0.009 A |
| Blush | 0.682 ± 0.005 A | 56.59 ± 1.88 C | 15.51 ± 6.64 C | 0.73 ± 0.017 C | |
| Starburst | 0.656 ± 0.003 B | 65.54 ± 0.75 B | 18.40 ± 1.90 B | 0.75 ± 0.008 C | |
| Purple | 0.641 ± 0.003 C | 50.51 ± 0.82 D | 38.39 ± 4.75 BC | 0.78 ± 0.009 B | |
| Moldy | 0.576 ± 0.013 D | 76.14 ± 2.64 A | 97.66 ± 38.54 A | 0.79 ± 0.038 BC | |
| ANOVA p | <0.0001 *** | <0.0001 *** | 0.0002 ** | <0.0001 *** | |
| B73 × | Asym. | 0.713 ± 0.007 A | 19.73 ± 1.36 C | 4.58 ± 0.92 AB | 0.91 ± 0.02 A |
| CML333 | Blush | 0.691 ± 0.005 B | 58.68 ± 2.20 B | 14.35 ± 5.01 AB | 0.74 ± 0.02 B |
| Starburst | 0.685 ± 0.006 B | 70.49 ± 1.49 A | 13.80 ± 3.12 A | 0.73 ± 0.02 B | |
| Purple | 0.658 ± 0.006 C | 54.04 ± 1.79 B | 31.19 ± 10.31 B | 0.71 ± 0.02 B | |
| Moldy | 0.601 ± 0.021 D | 77.95 ± 3.77 A | 85.22 ± 53.51 AB | 0.73 ± 0.07 B | |
| ANOVA p | <0.0001 *** | <0.0001 *** | 0.0427 * | 0.0001 ** | |
| B73 × | Asym. | 0.688 ± 0.005 A | 18.09 ± 0.68 D | 6.17 ± 0.70 A | 0.94 ± 0.01 A |
| CML52 | Blush | 0.606 ± 0.020 B | 46.78 ± 7.61 BC | 8.75 ± 8.17 AB | 0.70 ± 0.07 BC |
| Starburst | 0.660 ± 0.004 B | 59.81 ± 1.32 B | 10.46 ± 2.16 AB | 0.74 ± 0.01 C | |
| Purple | 0.655 ± 0.008 B | 41.05 ± 1.57 C | 21.21 ± 7.81 B | 0.78 ± 0.02 B | |
| Moldy | 0.503 ± 0.039 C | 84.19 ± 5.38 A | 32.09 ± 24.56 AB | 0.66 ± 0.08 BC | |
| ANOVA p | <0.0001 *** | <0.0001 *** | 0.113 | <0.0001 *** | |
| B73 × | Asym. | 0.693 ± 0.004 A | 21.47 ± 0.79 C | 14.70 ± 4.11 AB | 0.99 ± 0.01 A |
| CML69 | Blush | 0.659 ± 0.016 AB | 46.36 ± 6.61 B | 39.96 ± 25.15 BC | 0.78 ± 0.07 BC |
| Starburst | 0.657 ± 0.005 B | 67.74 ± 1.40 A | 21.59 ± 4.09 C | 0.75 ± 0.02 C | |
| Purple | 0.644 ± 0.006 B | 54.22 ± 1.63 B | 42.20 ± 8.61 C | 0.78 ± 0.02 BC | |
| Moldy | 0.588 ± 0.021 C | 72.49 ± 5.74 A | 179.86 ± 116.91 A | 0.88 ± 0.07 AB | |
| ANOVA p | <0.0001 *** | <0.0001 *** | 0.015 * | <0.0001 *** | |
| B73 × | Asym. | 0.692 ± 0.006 A | 20.88 ± 0.85 D | 21.91 ± 7.24 A | 0.97 ± 0.02 A |
| NC358 | Blush | 0.654 ± 0.016 AB | 56.06 ± 4.60 BC | 4.93 ± 1.84 B | 0.67 ± 0.05 D |
| Starburst | 0.617 ± 0.006 B | 66.45 ± 1.68 A | 29.82 ± 5.56 A | 0.77 ± 0.02 CD | |
| Purple | 0.623 ± 0.006 B | 51.19 ± 1.40 C | 53.31 ± 10.22 A | 0.82 ± 0.02 B | |
| Moldy | 0.562 ± 0.029 C | 68.74 ± 7.55 AB | 60.22 ± 31.84 A | 0.89 ± 0.08 ABC | |
| ANOVA p | <0.0001 *** | <0.0001 *** | 0.0502 | <0.0001 *** |
| B73 × CML333 Family | ||||
|---|---|---|---|---|
| rP/rG | BDENinoc | FER | FUM | FUM:FER |
| BDENinoc | −0.46 *** | −0.25 *** | −0.15 * | |
| FER | −0.53 *** | 0.17 ** | −0.18 ** | |
| FUM | −0.31 ** | 0.28 *** | 0.94 *** | |
| FUM:FER | 0.02 | −0.33*** | 0.80 *** | |
| B73 × CML52 family | ||||
| rP/rG | BDENinoc | FER | FUM | FUM:FER |
| BDENinoc | −0.47 *** | −0.12 * | 0.12 * | |
| FER | −0.48 *** | 0.08 ms | −0.36 *** | |
| FUM | −0.18 * | 0.20 * | 0.90 *** | |
| FUM:FER | 0.15 * | −0.46 *** | 0.75 *** | |
| B73 × CML69 family | ||||
| rP/rG | BDENinoc | FER | FUM | FUM:FER |
| BDENinoc | −0.55 *** | −0.30 *** | 0.02 | |
| FER | −0.53 *** | −0.01 | −0.33 *** | |
| FUM | −0.27 ** | 0.21** | 0.95 *** | |
| FUM:FER | 0.06 | −0.39 *** | 0.81 *** | |
| B73 × NC358 family | ||||
| rP/rG | BDENinoc | FER | FUM | FUM:FER |
| BDENinoc | −0.59 *** | −0.19 ** | 0.11 ms | |
| FER | −0.58 *** | 0.21 *** | −0.14 ** | |
| FUM | −0.25 ** | 0.12 | 0.94 *** | |
| FUM:FER | 0.15 ms | −0.46 *** | 0.79 *** | |
| Family | Trait | Random Effects (Variance Proportions) | Fixed Effect (p-Value) | H | ||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Env. | G*E | B[E] | Error | DTS | |||
| B73 × CML333 | BDENinoc | 0.64 | 0.01 | 0.04 | 0 | 0.31 | 0.1 | 0.79 |
| FER | 0.42 | 0.01 | 0.12 | 0 | 0.45 | 0.9 | 0.75 | |
| FUM | 0.02 | 0.61 | 0.16 | 0 | 0.21 | 0.3 | 0.12 | |
| FUM:FER | 0.03 | 0.58 | 0.10 | 0 | 0.29 | 0.6 | 0.17 | |
| Asymptomatic | 0.05 | 0.07 | 0.17 | 0.02 | 0.69 | 0.2 | 0.15 | |
| Blush | 0.18 | 0.13 | 0.30 | 0.01 | 0.38 | 0.004 ** | 0.44 | |
| Starburst | 0.12 | 0.18 | 0.17 | 0.01 | 0.53 | 0.9 | 0.33 | |
| Purple | 0.04 | 0.25 | 0.12 | 0.03 | 0.56 | 0.5 | 0.16 | |
| Moldy | 0.17 | 0.03 | 0.80 | 0 | 0.001 | 0.3 | 0.40 | |
| B73 × CML52 | BDENinoc | 0.33 | 0.003 | 0 | 0.04 | 0.62 | 0.002 ** | 0.52 |
| FER | 0.30 | 0.20 | 0.06 | 0.03 | 0.41 | 0.03 * | 0.72 | |
| FUM | 0.03 | 0.46 | 0.01 | 0.04 | 0.46 | 0.1 | 0.17 | |
| FUM:FER | 0.02 | 0.48 | 0 | 0.01 | 0.48 | 0.3 | 0.12 | |
| Asymptomatic | 0.06 | 0.27 | 0.17 | 0.03 | 0.48 | 0.8 | 0.21 | |
| Blush | 0 | 0.01 | 0 | 0 | 0.99 | 0.04 * | 0 | |
| Starburst | 0.11 | 0.28 | 0.18 | 0.02 | 0.42 | 0.6 | 0.35 | |
| Purple | 0.03 | 0.30 | 0.20 | 0.01 | 0.46 | 0.4 | 0.13 | |
| Moldy | 0.31 | 0.01 | 0.68 | 0 | 1 × 10−5 | 0.8 | 0.58 | |
| B73 × CML69 | BDENinoc | 0.46 | 0.03 | 0.32 | 0.02 | 0.18 | 0.04 * | 0.65 |
| FER | 0.22 | 0.14 | 0.18 | 0.03 | 0.43 | 0.8 | 0.59 | |
| FUM | 0.02 | 0.70 | 0.13 | 0.01 | 0.14 | 0.1 | 0.15 | |
| FUM:FER | 0.02 | 0.70 | 0.07 | 0.02 | 0.19 | 0.3 | 0.21 | |
| Asymptomatic | 0.09 | 0.13 | 0.16 | 0 | 0.62 | 0.2 | 0.25 | |
| Blush | 0.06 | 0.04 | 0.01 | 0 | 0.88 | 0.05 | 0.18 | |
| Starburst | 0.08 | 0.24 | 0.15 | 0.003 | 0.53 | 0.005 ** | 0.27 | |
| Purple | 0.07 | 0.26 | 0.21 | 0.01 | 0.46 | 0.03* | 0.24 | |
| Moldy | 0.17 | 0.03 | 0.80 | 0 | 8 × 10−5 | 0.009 ** | 0.39 | |
| B73 × NC358 | BDENinoc | 0.23 | 0.08 | 0.56 | 0.02 | 0.12 | 0.07 | 0.40 |
| FER | 0.27 | 0.22 | 0.06 | 0.04 | 0.40 | 0.003 ** | 0.70 | |
| FUM | 0.01 | 0.73 | 0.04 | 0 | 0.22 | 0.7 | 0.14 | |
| FUM:FER | 0.03 | 0.65 | 0.06 | 0 | 0.27 | 0.7 | 0.19 | |
| Asymptomatic | 0.05 | 0.04 | 0 | 0.05 | 0.86 | 0.08 | 0.15 | |
| Blush | 0.11 | 0.02 | 0.87 | 0 | 0.001 | 0.9 | 0.28 | |
| Starburst | 0.06 | 0.20 | 0.03 | 0.01 | 0.70 | 0.003 ** | 0.21 | |
| Purple | 0.07 | 0.25 | 0 | 0.01 | 0.67 | 0.03 * | 0.24 | |
| Moldy | 0.13 | 0.01 | 0.85 | 0 | 0.002 | 0.4 | 0.32 | |
| Analysis | Chr. | Position (bp) | Associated Traits | Marker Effect on Trait (Non-B73 vs. B73 Allele) |
|---|---|---|---|---|
| Joint | 1 | 17,256,105– | BDENinoc | +0.003CML333; +0.013CML52; +0.019CML69; −0.001NC358 |
| family | 25,068,060 | Blush | +0.054CML333; +0.002CML52; −0.001CML69; +0.007NC358 | |
| CobLen | −4.874CML333; −3.899CML52; −5.097CML69; −13.611NC358 | |||
| 2 | 20,081,914– | Blush | +0.073CML333; +0.001CML52; +0.012CML69; +0.005NC358 | |
| 50,634,516 | CobDiam | +0.531CML333; +0.380CML52; +0.510CML69; +0.907NC358 | ||
| CobLen | +3.033CML333; +6.651CML52; +3.954CML69; +9.236NC358 | |||
| CobMass | +1.197CML333; +0.655CML52; +1.605CML69; +1.843NC358 | |||
| CobVol | +5.456CML333; +3.441CML52; +4.325CML69; +8.825NC358 | |||
| Moldy | −0.026CML333; −0.013CML52; −0.021CML69; −0.006NC358 | |||
| Starburst | −0.001CML333; +0.023CML52; +0.044CML69; +0.047NC358 | |||
| 2 | 193,400,572– | BDENinoc | +0.025CML333; +0.003CML52; +0.010CML69; +0.002NC358 | |
| 196,813,641 | CobMass | −1.545CML333; +0.418CML52; −1.314CML69; +0.220NC358 | ||
| 5 | 205,258,663– | BDENinoc | +0.009CML333; −0.016CML52; −0.002CML69; −0.016NC358 | |
| 208,297,027 | Blush | −0.088CML333; −0.001CML52; −0.006CML69; −0.017NC358 | ||
| CobLen | −0.868CML333; −2.017CML52; −7.233CML69; −2.325NC358 | |||
| FER | −0.606CML333; +5.843CML52; −1.058CML69; +8.576NC358 | |||
| 6 | 130,721,405– | Asym | +0.002CML333; +0.027CML52; +0.015CML69; +0.007NC358 | |
| 133,795,910 | FER | −4.192CML333; −6.906CML52; −6.253CML69; −4.148NC358 | ||
| 10 | 79,389,587– | CobDen | +0.005CML333; +0.012CML52; +0.0003CML69; +0.007NC358 | |
| 116,043,385 | Starburst | −0.031CML333; −0.037CML52; −0.029CML69; −0.048NC358 | ||
| B73 × | 1 | 19,128,807– | Blush | +0.052CML333 |
| CML333 | 24,311,471 | CobLen | −5.937CML333 | |
| 1 | 47,813,030– | FER | +6.660CML333 | |
| 57,347,172 | FUM | +0.162CML333 | ||
| 2 | 21,767,606– | BDENinoc | +0.020CML333 | |
| 35,311,519 | Blush | +0.065CML333 | ||
| 7 | 4,823,956– | CobLen | +6.465CML333 | |
| 6,178,479 | CobVol | +15.265CML333 | ||
| Starburst | +0.046CML333 | |||
| 7 | 14,152,199– | FER | +4.985CML333 | |
| 23,748,237 | FUM:FER | −0.009CML333 | ||
| 8 | 130,983,626– | BDENinoc | +0.015CML333 | |
| 151,773,181 | CobDen | +0.010CML333 | ||
| CobDiam | −1.587CML333 | |||
| Starburst | +0.040CML333 | |||
| B73 × | 5 | 83,007,601– | BDENinoc | +0.018CML52 |
| CML52 | 140,575,190 | BDENuninoc | +0.009CML52 | |
| 6 | 129,994,297– | Asym | +0.027CML52 | |
| 140,630,780 | CobDiam | −0.744CML52 | ||
| 6 | 154,530,689– | FER | −7.612CML52 | |
| 156,104,230 | FUM:FER | +0.010CML52 | ||
| 8 | 132,405,651– | BDENuninoc | +0.010CML52 | |
| 146,365,593 | CobVol | −6.924CML52 | ||
| FER | −5.718CML52 | |||
| B73 × | 2 | 49,846,838– | CobMass | +2.016CML69 |
| CML69 | 87,089,680 | Moldy | −0.023CML69 | |
| Starburst | +0.048CML69 | |||
| 4 | 17,330,826– | BDENinoc | −0.018CML69 | |
| 21,710,411 | FER | +6.347CML69 | ||
| 4 | 167,066,431– | Purple | +0.034CML69 | |
| 170,804,182 | Starburst | −0.050CML69 | ||
| 7 | 138,072,861– | Asym | +0.020CML69 | |
| 142,429,440 | FER | −7.435CML69 | ||
| B73 × | 2 | 144,159,847– | Purple | −0.031NC358 |
| NC358 | 160,579,525 | Starburst | +0.046NC358 | |
| 3 | 6,427,177– | BDENinoc | +0.021NC358 | |
| 8,563,589 | Starburst | −0.054NC358 | ||
| 10 | 16,505,881– | Asym | +0.018NC358 | |
| 77,678,052 | CobDen | +0.009NC358 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, L.; Zila, C.T.; Moreta Mejía, D.E.; Montoya Arbelaez, M.; Balint-Kurti, P.J.; Holland, J.B.; Nelson, R.J. Diverse Components of Resistance to Fusarium verticillioides Infection and Fumonisin Contamination in Four Maize Recombinant Inbred Families. Toxins 2019, 11, 86. https://doi.org/10.3390/toxins11020086
Morales L, Zila CT, Moreta Mejía DE, Montoya Arbelaez M, Balint-Kurti PJ, Holland JB, Nelson RJ. Diverse Components of Resistance to Fusarium verticillioides Infection and Fumonisin Contamination in Four Maize Recombinant Inbred Families. Toxins. 2019; 11(2):86. https://doi.org/10.3390/toxins11020086
Chicago/Turabian StyleMorales, Laura, Charles T. Zila, Danilo E. Moreta Mejía, Melissa Montoya Arbelaez, Peter J. Balint-Kurti, James B. Holland, and Rebecca J. Nelson. 2019. "Diverse Components of Resistance to Fusarium verticillioides Infection and Fumonisin Contamination in Four Maize Recombinant Inbred Families" Toxins 11, no. 2: 86. https://doi.org/10.3390/toxins11020086
APA StyleMorales, L., Zila, C. T., Moreta Mejía, D. E., Montoya Arbelaez, M., Balint-Kurti, P. J., Holland, J. B., & Nelson, R. J. (2019). Diverse Components of Resistance to Fusarium verticillioides Infection and Fumonisin Contamination in Four Maize Recombinant Inbred Families. Toxins, 11(2), 86. https://doi.org/10.3390/toxins11020086