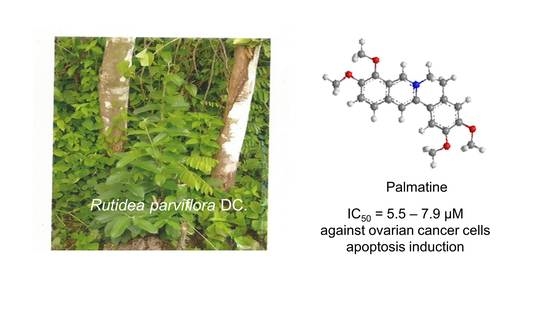
Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells †
Abstract
:1. Introduction
2. Results
2.1. Bioassay-Guided Isolation and Identification of Palmatine
2.2. Apoptosis Studies
2.2.1. Caspase 3/7 Activity and Western Blotting Analysis
2.2.2. Flow Cytometric Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Extraction of Plant Materials
4.3. Analysis of the Bioactive Fraction of R. parviflora by Gas Chromatography–Mass Spectrometry (GC–MS)
4.4. Liquid Chromatography Mass Spectrometry (LC-MS) Analysis
4.5. NMR Spectroscopy
4.6. Purification and Isolation of Bioactive Compounds
4.7. Cell Culture
4.8. Cell Growth Assay
4.9. Caspase 3/7 Activity
4.10. Western Blotting
4.11. Flow Cytometry
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer (accessed on 23 April 2019).
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar]
- Bowtell, D.D.; Bohm, S.; Ahmed, A.A.; Aspuria, P.J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Petty, R.; Evans, A.; Duncan, I.; Kurbacher, C.; Cree, I. Drug resistance in ovarian cancer—The role of p53. Pathol. Oncol. Res. 1998, 4, 97–102. [Google Scholar] [CrossRef]
- Vasey, P.A. Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br. J. Cancer 2003, 89, S23–28. [Google Scholar] [CrossRef]
- Binju, M.; Padilla, M.A.; Singomat, T.; Kaur, P.; Suryo Rahmanto, Y.; Cohen, P.A.; Yu, Y. Mechanisms underlying acquired platinum resistance in high grade serous ovarian cancer—A mini review. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 371–378. [Google Scholar] [CrossRef]
- Franzese, E.; Centonze, S.; Diana, A.; Carlino, F.; Guerrera, L.P.; Di Napoli, M.; De Vita, F.; Pignata, S.; Ciardiello, F.; Orditura, M. PARP inhibitors in ovarian cancer. Cancer Treat. Rev. 2019, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sowemimo, A.; Van de Venter, M.; Baatjies, L.; Koekemoer, T. Cytotoxicity of some Nigerian plants used in traditional cancer treatment. Planta Med. 2010, 76, 1224–1225. [Google Scholar] [CrossRef]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.N.; Varoni, E.M.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in cancer invasion and metastasis. Phytother. Res. 2018, 32, 1425–1449. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Johnson-Ajinwo, O.R.; Uche, F.I. Potential of phytochemicals and their derivatives in the treatment of ovarian cancer. In Handbook on Ovarian Cancer: Risk Factors, Therapies and Prognosis; Collier, B.C., Ed.; Nova Science publishers: Hauppauge, NY, USA, 2015. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Uche, F.I.; Li, W.W.; Richardson, A.; Greenhough, T.J. Anticancer activities of cyclotides from Viola yedeonsis Makino (Violaceae). Planta Med. 2014, 80, 818. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Cytotoxic effects of stem bark extracts and pure compounds from Margaritaria discoidea on human ovarian cancer cell lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Identification and evaluation of anticancer compounds from three Nigerian plants used in traditional medicines. Biochem. Pharmacol. 2017, 139, 128. [Google Scholar] [CrossRef]
- Uche, F.; Li, W.W.; Richardson, A.; Greenhough, T.J. Anti-ovarian cancer activities of alkaloids from Triclisia subcordata olive (Menispermecaea). Planta Med. 2014, 80, 813. [Google Scholar] [CrossRef]
- Uche, F.I.; Drijfhout, F.; McCullagh, J.; Richardson, A.; Li, W.W. Cytotoxicity effects and apoptosis induction by cycleanine and tetrandrine. Planta Med. 2016, 82. [Google Scholar] [CrossRef]
- Uche, F.I.; Drijfhout, F.P.; McCullagh, J.; Richardson, A.; Li, W.W. Cytotoxicity Effects and Apoptosis Induction by Bisbenzylisoquinoline Alkaloids from Triclisia subcordata. Phytother. Res. 2016, 30, 1533–1539. [Google Scholar] [CrossRef]
- Uche, F.I.; Abed, M.; Abdullah, M.I.; Drijfhout, F.P.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.W. Isolation, identification and anti-cancer activity of minor alkaloids from Triclisia subcordata Oliv. Biochem. Pharmacol. 2017, 139, 112. [Google Scholar] [CrossRef]
- Uche, F.I.; Abed, M.N.; Abdullah, M.I.; Drijfhout, F.P.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.W. Isochondodendrine and 2 ’-norcocsuline: additional alkaloids from Triclisia subcordata induce cytotoxicity and apoptosis in ovarian cancer cell lines. Rsc Adv. 2017, 7, 44154–44161. [Google Scholar] [CrossRef]
- Uche, F.I.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.W. Synthesis of (aminoalkyl)cycleanine analogues: Cytotoxicity, cellular uptake, and apoptosis induction in ovarian cancer cells. Bioorg. Med. Chem. Lett. 2018, 28, 1652–1656. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Ullah, I.; Mbye, H.; Richardson, A.; Horrocks, P.; Li, W.W. The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Bioorg. Med. Chem. Lett. 2018, 28, 1219–1222. [Google Scholar] [CrossRef]
- Burkill, H.M. The useful plants of west tropical Africa; Royal Botanic Gardens: London, UK, 1985. [Google Scholar]
- Thanga Krishna Kumari, S.; Muthukumarasamy, S.; Mohan, V.R. GC-MS determination of bioactive components of Canscora perfoliata Lam. (Gentianaceae). J. Appl. Pharmal. Sci. 2012, 2, 210–214. [Google Scholar]
- Whitacre, C.M.; Zborowska, E.; Willson, J.K.; Berger, N.A. Detection of poly(ADP-ribose) polymerase cleavage in response to treatment with topoisomerase I inhibitors: A potential surrogate end point to assess treatment effectiveness. Clin. Cancer Res. 1999, 5, 665–672. [Google Scholar]
- Trucco, C.; Oliver, F.J.; de Murcia, G.; Menissier-de Murcia, J. DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucleic Acids Res. 1998, 26, 2644–2649. [Google Scholar] [CrossRef] [Green Version]
- D’Amours, D.; Germain, M.; Orth, K.; Dixit, V.M.; Poirier, G.G. Proteolysis of poly(ADP-ribose) polymerase by caspase 3: kinetics of cleavage of mono(ADP-ribosyl)ated and DNA-bound substrates. Radiat. Res. 1998, 150, 3–10. [Google Scholar] [CrossRef]
- Ding, P.L.; Chen, L.Q.; Lu, Y.; Li, Y.G. Determination of protoberberine alkaloids in Rhizoma Coptidis by ERETIC (1)H NMR method. J. Pharm. Biomed. Anal. 2012, 60, 44–50. [Google Scholar] [CrossRef]
- Hambright, H.G.; Batth, I.S.; Xie, J.; Ghosh, R.; Kumar, A.P. Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFkappaB/FLIP. Mol. Carcinog. 2015, 54, 1227–1234. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Zhang, N.; Xue, C.; Leung, A.W.; Zhang, H.; Tang, Q.J.; Xu, C. Palmatine hydrochloride mediated photodynamic inactivation of breast cancer MCF-7 cells: Effectiveness and mechanism of action. Photodiagnosis Photodyn. Ther. 2016, 15, 133–138. [Google Scholar] [CrossRef]
- Chakravarthy, D.; Munoz, A.R.; Su, A.; Hwang, R.F.; Keppler, B.R.; Chan, D.E.; Halff, G.; Ghosh, R.; Kumar, A.P. Palmatine suppresses glutamine-mediated interaction between pancreatic cancer and stellate cells through simultaneous inhibition of survivin and COL1A1. Cancer Lett. 2018, 419, 103–115. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Zhang, N.; Xue, C.; Leung, A.W.; Zhang, H.; Xu, C.; Tang, Q.J. Photodynamic action of palmatine hydrochloride on colon adenocarcinoma HT-29 cells. Photodiagnosis Photodyn. Ther. 2016, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Padmapriya, K.; Barthwal, R. Structural and biophysical insight into dual site binding of the protoberberine alkaloid palmatine to parallel G-quadruplex DNA using NMR, fluorescence and Circular Dichroism spectroscopy. Biochimie 2018, 147, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.K.; Li, H.; Dong, C.L.; He, X.; Guo, C.R.; Zhang, C.F.; Yu, C.H.; Wang, C.Z.; Yuan, C.S. Palmatine from Mahonia bealei attenuates gut tumorigenesis in ApcMin/+ mice via inhibition of inflammatory cytokines. Mol. Med. Rep. 2016, 14, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCloud, T.G. High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules 2010, 15, 4526–4563. [Google Scholar] [CrossRef]
- Li, W.W.; Barz, W. Structure and accumulation of phenolics in elicited Echinacea purpurea cell cultures. Planta Med. 2006, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Barz, W. Biotechnological production of two new 8,4’-oxynorneolignans by elicitation of Echinacea purpurea cell cultures. Tetrahedron Lett. 2005, 46, 2973–2977. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Tong, R.B. A General, Concise Strategy that Enables Collective Total Syntheses of over 50 Protoberberine and Five Aporhoeadane Alkaloids within Four to Eight Steps. Chem.-Eur. J. 2016, 22, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.T.; Kao, C.L.; Huang, S.C.; Chen, C.T.; Li, H.T.; Chen, C.Y. Secondary Metabolites from the Stems of Mahonia oiwakensis. Chem. Nat. Compd. 2017, 53, 997–998. [Google Scholar] [CrossRef]
- Shah, B.A.; Kumar, A.; Gupta, P.; Sharma, M.; Sethi, V.K.; Saxena, A.K.; Singh, J.; Qazi, G.N.; Taneja, S.C. Cytotoxic and apoptotic activities of novel amino analogues of boswellic acids. Bioorg. Med. Chem. Lett. 2007, 17, 6411–6416. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]



| Extract, Fraction and Compounds | OVCAR-4 | OVCAR-8 | A2780 | A2780cis | HOE |
|---|---|---|---|---|---|
| (µg/ml) | |||||
| Organic extract | 6.6 ± 1.6 | 8.7 ± 0.5 | 3.2 ± 0.3 | n.d. | n.d. |
| Aqueous extract | n.d. | 5.9 ± 0.03 | 2.2 ± 0.5 | 3.7 ± 0.03 | n.d. |
| n-Hexane fraction | 23.3 ± 1.0 | 18.3 ± 0.3 | 7.3 ± 0.8 | n.d. | n.d. |
| Ethyl acetate fraction | 5.4 ± 0.3 | 5.8 ± 0.4 | 2.5 ± 0.2 | n.d. | n.d. |
| n-Butanol fraction | 2.6 ± 0.1 | 2.6 ± 0.3 | 1.7 ± 0.2 | n.d. | n.d. |
| Aqueous fraction | 22.9 ± 1.3 | 22.1 ± 1.1 | 12.8 ± 1.3 | n.d. | n.d. |
| (µM) | |||||
| Palmatine (1) | 7.4 ± 0.3 | 7.9 ± 0.5 | 6.6 ± 0.5 | 5.5 ± 0.9 | 25.1 ± 5.0 |
| SI for 1 | 3.4 | 3.2 | 3.8 | 4.6 | - |
| Urs-12-en-24-oic acid, 3-oxo-, methyl ester (2) | 85.4 ± 2.4 | 48.9 ± 2.0 | 31.6 ± 3.3 | n.d. | >200 |
| SI for 2 | >2 | >4 | >6.5 | - | - |
| Carboplatin | 11.1 ± 0.4 | 10.8 ± 1.3 | 16.0 ± 1.0 | >100 | 15.2 ± 3.0 |
| SI for carboplatin | 1.4 | 1.4 | 0.95 | <0.15 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.-W. Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins 2019, 11, 237. https://doi.org/10.3390/toxins11040237
Johnson-Ajinwo OR, Richardson A, Li W-W. Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins. 2019; 11(4):237. https://doi.org/10.3390/toxins11040237
Chicago/Turabian StyleJohnson-Ajinwo, Okiemute Rosa, Alan Richardson, and Wen-Wu Li. 2019. "Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells" Toxins 11, no. 4: 237. https://doi.org/10.3390/toxins11040237
APA StyleJohnson-Ajinwo, O. R., Richardson, A., & Li, W. -W. (2019). Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins, 11(4), 237. https://doi.org/10.3390/toxins11040237