Using Advanced Spectroscopy and Organic Matter Characterization to Evaluate the Impact of Oxidation on Cyanobacteria
Abstract
:1. Introduction
2. Results and Discussion
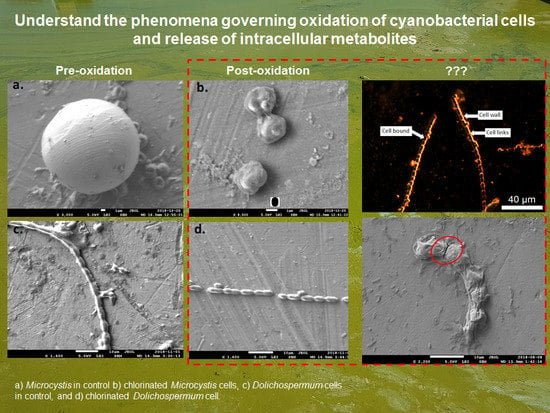
2.1. Cell Viability Post-Oxidation
2.2. LC-OCD-OND
2.3. EDM/HSI
3. Conclusions
4. Materials and Methods
4.1. Cyanobacteria Culture Sample Preparation
4.2. Preparation of Oxidants and Calculation of Exposure
4.3. DOC and LC-OCD-OND
4.4. Cell Counts, Morphology and Integrity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Elliott, J.A. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012, 46, 1364–1371. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Watson, S. Aquatic taste and odor: A primary signal of drinking water integrity. J. Toxicol. Environ. Health A 2004, 67, 1779–1795. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, N.; Hill, D.J.; Diggs, D.L.; Faison, B.D.; Francis, B.M.; Lang, J.R.; Larue, M.M.; Le, T.-T.; Loftin, K.A.; Lugo, J.N.; et al. A critical review of the postulated role of the nonessential amino acid, b-N-methylamino-L- alanine, in neurodegenerative disease in humans. J. Toxicol. Environ. Health 2017, 20, 183–229. [Google Scholar] [CrossRef]
- Lévesque, B.; Gervais, M.; Chevalier, P.; Gauvin, D.; Anassour-Laouan-Sidi, E.; Gingras, S.; Fortin, N.; Brisson, G.; Greer, C.; Bird, D. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci. Total Environ. 2014, 466, 397–403. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Recommendations for Public Water Systems to Manage Cyanotoxins in Drinking Water; Office of Water: Washington, DC, USA, 2015; EPA 815-r-15-010.
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; World Health Organization (WHO): London, UK, 1999. [Google Scholar]
- Health Canada. Cyanobacteria Toxins in Drinking Water. 2016. Available online: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/health-system-systeme-sante/consultations/cyanobacteria-cyanobacterie/alt/cyanobacteria-cyanobacterie-eng.pdf (accessed on 19 February 2019).
- Zamyadi, A.; MacLeod, S.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Henderson, R.; Stuetz, R.; Hofmann, R.; Ho, L.; Newcombe, G. Fate of geosmin and 2-methylisoborneol in full-scale water treatment plants. Water Res. 2015, 83, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Pazouki, P.; Prevost, M.; McQuiad, N.; Barbeau, B.; de Boutray, M.-L.; Zamyadi, A.; Dorner, S. Breakthrough of cyanobacteria in bank filtration. Water Res. 2016, 102, 170–179. [Google Scholar] [CrossRef]
- Zamyadi, A.; Dorner, S.; Ndong, M.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Low-risk cyanobacterial bloom sources: Cell accumulation within full-scale treatment plants. J. Am. Water Works Assoc. 2013, 102, e651–e663. [Google Scholar] [CrossRef]
- Almuhtaram, H.; Cui, Y.; Zamyadi, A.; Hofmann, R. Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source. Toxins 2018, 10, 430. [Google Scholar] [CrossRef]
- Pestana, C.J.; Reeve, P.J.; Sawade, E.; Voldoire, C.F.; Newton, K.; Praptiwi, R.; Collingnon, L.; Dreyfus, J.; Hobson, P.; Gaget, V.; et al. Fate of cyanobacteria in drinking water treatment plant lagoon supernatant and sludge. Sci. Total Environ. 2016, 565, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Naceradska, J.; Kopecka, I.; Baresova, M.; Jefferson, B.; Li, X.; Henderson, R. The impact of algogenic organic matter on water treatment plant operation and water quality: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 291–335. [Google Scholar] [CrossRef]
- Fan, J.J.; Daly, R.; Hobson, P.; Ho, L.; Brookes, J. Impact of potassium permanganate on cyanobacterial cell integrity and toxin release and degradation. Chemosphere 2013, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Ho, L.; Hobson, P.; Daly, R.; Brookes, J. Application of various oxidants for cyanobacteria control and cyanotoxin removal in wastewater treatment. J. Environ. Eng. 2014, 140, 4022–4028. [Google Scholar] [CrossRef]
- Coral, L.A.; Zamyadi, A.; Barbeau, B.; Bassetti, F.J.; Lapolli, F.R.; Prévost, M. Oxidation of M. aeruginosa and A. flosaquae by ozone: Impacts on cell integrity and chlorination by-product formation. Water Res. 2013, 47, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shi, H.; Timmons, T.; Adams, C. Release and removal of microcystins from microcystis during oxidative-, physical-, and UV-based disinfection. J. Environ. Eng. 2009, 136, 2–11. [Google Scholar] [CrossRef]
- Zamyadi, A.; Coral, L.A.; Barbeau, B.; Dorner, S.; Lapolli, F.R.; Prévost, M. Fate of toxic cyanobacterial genera from natural bloom events during ozonation. Water Res. 2015, 7373, 204–215. [Google Scholar] [CrossRef]
- Fan, J.; Rao, L.; Chiu, Y.T.; Lin, T.F. Impact of chlorine on the cell integrity and toxin release and degradation of colonial Microcystis. Water Res. 2016, 102, 394–404. [Google Scholar] [CrossRef]
- He, X.X.; Wert, E.C. Colonial cell disaggregation and intracellular microcystin release following chlorination of naturally occurring Microcysis. Water Res. 2016, 101, 10–16. [Google Scholar] [CrossRef]
- Zamyadi, A.; Fan, Y.; Daly, R.I.; Prévost, M. Chlorination of Microcystis aeruginosa: Toxin release and oxidation, cellular chlorine demand and disinfection by-products formation. Water Res. 2013, 47, 1080–1090. [Google Scholar] [CrossRef]
- Jones, C.T. Determination of the kinetic constants of two consecutive first-order reactions. Biochem. J. 1970, 118, 810–812. [Google Scholar] [CrossRef]
- Zamyadi, A.; Romanis, C.; Mills, T.; Neilan, B.; Choo, F.; Coral, L.A.; Gale, D.; Newcombe, G.; Crosbie, N.; Stuetz, R.; et al. Diagnosing water treatment critical control points for cyanobacterial removal: Exploring benefits of combined microscopy, next-generation sequencing, and cell integrity methods. Water Res. 2019, 152, 96–105. [Google Scholar] [CrossRef]
- Zhang, H.; Dan, Y.; Adams, C.D.; Shi, H.; Ma, Y.; Eichholz, T. Effect of oxidant demand on the release and degradation of Microcystin-LR from Microcystis Aeruginosa during oxidation. Chemosphere 2017, 181, 562–568. [Google Scholar] [CrossRef]
- Zamyadi, A.; Henderson, R.K.; Stuetz, R.; Newcombe, G.; Newtown, K.; Gladman, B. Cyanobacterial management in full-scale water treatment and recycling processes: Reactive dosing following intensive monitoring. Environ. Sci. Water Res. Technol. 2016, 2, 362–375. [Google Scholar] [CrossRef]
- Daly, R.I.; Ho, L.; Brookes, J.D. Effect of chlorination on Microcystis aeruginosa cell integrity and subsequent microcystin release and degradation. Environ. Sci. Technol. 2007, 41, 4447–4453. [Google Scholar] [CrossRef]
- Wert, E.C.; Dong, M.M.; Rosario-Ortiz, F.L. Using digital flow cytometry to assess the degradation of three cyanobacteria species after oxidation processes. Water Res. 2013, 47, 3752–3761. [Google Scholar] [CrossRef]
- Plummer, J.; Edzwald, J.K. Effects of chlorine and ozone on algal cell properties and removal of algae by coagulation. J. Water Supply Res.Technol.—AQUA 2002, 51, 307–318. [Google Scholar] [CrossRef]
- Huang, W.-J.; Cheng, B.-L.; Hu, S.-K.; Chu, C. Ozonation of Algae and Odor Causing Substances in Eutrophic Waters. J. Environ. Sci. Health Part A 2007, 41, 1587–1605. [Google Scholar] [CrossRef]
- Paine, E.C.; Slonecker, E.T.; Simon, N.S.; Rosen, B.H.; Resmini, R.G.; Allen, D.W. Optical characterization of two cyanobacteria genera, Aphanizomenon and Microcystis, with hyperspectral microscopy. J. Appl. Remote Sens. 2018, 12. [Google Scholar] [CrossRef]
- Randolph, K.; Wilson, J.; Tedesco, L.; Li, L.; Pascual, D.L.; Soyeux, E. Hyperspectral remote sensing of cyanobacteria in turbid productive water using optically active pigments, chlorophyll a and phycocyanin. Remote Sens. Environ. 2008, 112, 4009–4019. [Google Scholar] [CrossRef]
- Simis, S.G.; Peters, S.W.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Vincent, R.K.; Qin, X.; McKay, R.M.L.; Miner, J.; Czajkowski, K.; Savino, J.; Bridgeman, T. Phycocyanin detection from LANDSAT TM data for mapping cyanobacterial blooms in Lake Erie. Remote Sens. Environ. 2004, 89, 381–392. [Google Scholar] [CrossRef]
- Mikula, P.; Zezulka, S.; Jancula, D.; Marsalek, B. Metabolic activity and membrane integrity changes in Microcystis aeruginosa–new findings on hydrogen peroxide toxicity in cyanobacteria. Eur. J. Phycol. 2012, 47, 195–206. [Google Scholar] [CrossRef]
- Lin, T.F.; Chang, D.W.; Lien, S.K.; Tseng, Y.S.; Chiu, Y.T.; Wang, Y.S. Effect of chlorination on the cell integrity of two noxious cyanobacteria and their releases of odorants. J. Water Supply Res. Technol. AQUA 2009, 58, 539–551. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Ekowati, Y.; Neu, T.R.; Kleijn, J.M.; Winters, H.; Amy, G.; Schippers, J.C.; Kennedy, M.D. Characterization of algal organic matter produced by bloom-forming marine and freshwater algae. Water Res. 2015, 73, 216–230. [Google Scholar] [CrossRef]
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae. Water Res. 2010, 44, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography–organic carbon detection–organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorption as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Meylan, S.; Salhi, E.; Koster, O.; Egli, T.; Von Gunten, U. Formation of assimilable organic carbon (AOC) and specific natural organic matter (NOM) fractions during ozonation of phytoplankton. Water Res. 2007, 41, 1447–1454. [Google Scholar] [CrossRef]
- Laszakovits, J.R.; MacKay, A.A. Removal of cyanotoxins by potassium permanganate: Incorporating competition from natural water constituents. Water Res. 2019, 155, 86–95. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Planas, C.; Guadayol, J.M.; Droguet, M.; Escalas, A.; Rivera, J.; Caixach, J. Degradation of polyethoxylated nonylphenols in a sewage treatment plant. Quantitative analysis by isotopic dilution-HRGC/MS. Water Res. 2002, 36, 982–988. [Google Scholar] [CrossRef]
- Baker, P.D.; Fabbro, L.D. A Guide to the Identification of Common Blue-Green Algae (Cyanoprokaryotes) in Australian Freshwaters; Cooperative Research Centre for Freshwater Ecology: Canberra, Australia, 2002. [Google Scholar]
- Buskey, E.J.; Hyatt, C.J. Use of the FlowCAM for semi- automated recognition and, enumeration of red tide cells (Karenia brevis) in natural plankton samples. Harmful Algae 2006, 5, 685–692. [Google Scholar] [CrossRef]
- Théoret, T.; Wilkinson, K.J. Evaluation of enhanced darkfield microscopy and hyperspectral analysis to analyse the fate of silver nanoparticles in wastewaters. Anal. Methods 2017, 9, 3920–3928. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradinejad, S.; Glover, C.M.; Mailly, J.; Seighalani, T.Z.; Peldszus, S.; Barbeau, B.; Dorner, S.; Prévost, M.; Zamyadi, A. Using Advanced Spectroscopy and Organic Matter Characterization to Evaluate the Impact of Oxidation on Cyanobacteria. Toxins 2019, 11, 278. https://doi.org/10.3390/toxins11050278
Moradinejad S, Glover CM, Mailly J, Seighalani TZ, Peldszus S, Barbeau B, Dorner S, Prévost M, Zamyadi A. Using Advanced Spectroscopy and Organic Matter Characterization to Evaluate the Impact of Oxidation on Cyanobacteria. Toxins. 2019; 11(5):278. https://doi.org/10.3390/toxins11050278
Chicago/Turabian StyleMoradinejad, Saber, Caitlin M. Glover, Jacinthe Mailly, Tahere Zadfathollah Seighalani, Sigrid Peldszus, Benoit Barbeau, Sarah Dorner, Michèle Prévost, and Arash Zamyadi. 2019. "Using Advanced Spectroscopy and Organic Matter Characterization to Evaluate the Impact of Oxidation on Cyanobacteria" Toxins 11, no. 5: 278. https://doi.org/10.3390/toxins11050278
APA StyleMoradinejad, S., Glover, C. M., Mailly, J., Seighalani, T. Z., Peldszus, S., Barbeau, B., Dorner, S., Prévost, M., & Zamyadi, A. (2019). Using Advanced Spectroscopy and Organic Matter Characterization to Evaluate the Impact of Oxidation on Cyanobacteria. Toxins, 11(5), 278. https://doi.org/10.3390/toxins11050278