Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies
Abstract
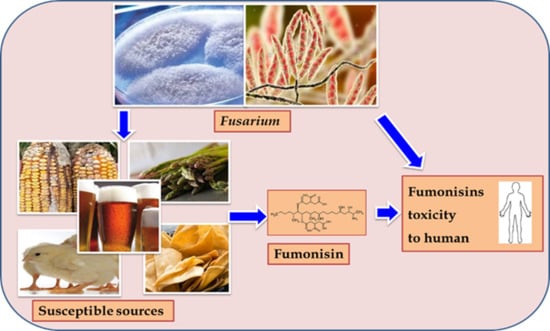
:1. Introduction
2. Major Source of Fumonisin
3. Chemistry and Biosynthesis of Fumonisin
4. Genes Responsible for Fumonisin Production
5. Occurrence in Food
6. Effects on Agriculture and Food
7. Mechanism of Toxicity and Health Effects of Fumonisins
7.1. Mechanism of Toxicity
7.2. Health Effects of Fumonisin
8. Effects of Processing on Fumonisin
9. Effects of Environmental Temperature on Fumonisin Production
10. Detection Techniques
11. Masked Mycotoxins as a major concern in detection
12. Degradation Kinetics
13. Management and Control Strategies
13.1. Management and Control using Agricultural Practices
13.2. Management and Control using Mycotoxin Binder
14. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rheeder, J.P.; Marasas, W.F.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Astoreca, A.; Magnoli, C.; Barberis, C.; Chiacchiera, S.; Combina, M.; Dalcero, A. Ochratoxin a production in relation to ecophysiological factors by Aspergillus section Nigri strains isolated from different substrates in Argentina. Sci. Total Environ. 2007, 388, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Astoreca, A.; Magnoli, C.; Ramirez, M.L.; Combina, M.; Dalcero, A. Water activity and temperature effects on growth of Aspergillus niger, A. awamori and A. carbonarius isolated from different substrates in Argentina. Int. J. Food Microbiol. 2007, 119, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, J.M.; Frisvad, J.C.; Thrane, U.; Nielsen, K.F. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J. Agric. Food Chem. 2009, 58, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Battilani, P. Fumonisins and their modified forms, a matter of concern in future scenario? World Mycotoxin J. 2016, 9, 727–739. [Google Scholar] [CrossRef]
- Cendoya, E.; Chiotta, M.L.; Zachetti, V.; Chulze, S.N.; Ramirez, M.L. Fumonisins and fumonisin-producing Fusarium occurrence in wheat and wheat by products: A review. J. Cereal Sci. 2018, 80, 158–166. [Google Scholar] [CrossRef]
- Braun, M.S.; Wink, M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 769–791. [Google Scholar] [CrossRef]
- Marasas, W.F. Fumonisins in Food; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Damiani, T.; Righetti, L.; Suman, M.; Galaverna, G.; Dall’Asta, C. Analytical issue related to fumonisins: A matter of sample comminution? Food Control 2019, 95, 1–5. [Google Scholar] [CrossRef]
- Shephard, G. Chromatographic determination of the fumonisin mycotoxins. J. Chromatogr. A 1998, 815, 31–39. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Orso, P.B.; Bordin, K.; Hara, R.V.; Luciano, F.B. Toxicological effects of fumonisin B1 in combination with other Fusarium toxins. Food Chem. Toxicol. 2018, 121, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Pezzani, C.H.; Pacin, A. Mycotoxins occurrence in Argentina’s maize (Zea mays L.), from 1999 to 2010. Food Control. 2012, 25, 660–665. [Google Scholar] [CrossRef]
- Vargas, E.; Preis, R.; Castro, L.; Silva, C. Co-occurrence of aflatoxins B 1, B 2, G 1, G 2, zearalenone and fumonisin B 1 in Brazilian corn. Food Addit. Contam. 2001, 18, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Zheng, H.; He, C.; Zhang, H. T-2 toxin, zearalenone and fumonisin B1 in feedstuffs from China. Food Addit. Contam. 2013, 6, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gutleb, A.; Caloni, F.; Giraud, F.; Cortinovis, C.; Pizzo, F.; Hoffmann, L.; Bohn, T.; Pasquali, M. Detection of multiple mycotoxin occurrences in soy animal feed by traditional mycological identification combined with molecular species identification. Toxicol. Rep. 2015, 2, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, I.; Martins, H.M.; Santos, S.; Costa, J.M.; Bernardo, F. Co-occurrence of mycotoxins in swine feed produced in Portugal. Mycotoxin Res. 2011, 27, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Ramos, A.J.; Marín, S.; Sanchis, V. Presence and co-occurrence of aflatoxins, deoxynivalenol, fumonisins and zearalenone in gluten-free and ethnic foods. Food Control 2012, 26, 282–286. [Google Scholar] [CrossRef]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control. 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Tansakul, N.; Jala, P.; Laopiem, S.; Tangmunkhong, P.; Limsuwan, S. Co-occurrence of five Fusarium toxins in corn-Dried Distiller’s Grains with Solubles in Thailand and comparison of ELISA and LC-MS/MS for fumonisin analysis. Mycotoxin Res. 2013, 29, 255–260. [Google Scholar] [CrossRef]
- Chu, F.S.; Li, G.Y. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 1994, 60, 847–852. [Google Scholar] [PubMed]
- Gelderblom, W.; Jaskiewicz, K.; Marasas, W.; Thiel, P.; Horak, R.; Vleggaar, R.; Kriek, N. Fumonisins--novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [PubMed]
- Carlson, D.B.; Williams, D.E.; Spitsbergen, J.M.; Ross, P.F.; Bacon, C.W.; Meredith, F.I.; Riley, R.T. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N′-nitro-nitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol. Appl. Pharmacol. 2001, 172, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Tang, L.; Lin, S.; Xue, K.S.; Mitchell, N.J.; Su, J.; Gelderblom, W.C.; Riley, R.T.; Phillips, T.D.; Wang, J.-S. Sequential dietary exposure to aflatoxin B1 and fumonisin B1 in F344 rats increases liver preneoplastic changes indicative of a synergistic interaction. Food Chem. Toxicol. 2016, 95, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, K.S.; Qian, G.; Lin, S.; Su, J.; Tang, L.; Gelderblom, W.C.; Riley, R.T.; Phillips, T.D.; Wang, J.-S. Modulation of pre-neoplastic biomarkers induced by sequential aflatoxin B1 and fumonisin B1 exposure in F344 rats treated with UPSN clay. Food Chem. Toxicol. 2018, 114, 316–324. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC), World Health Organisation (WHO). Fumonisin B1. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Press: Lyon, France, 2002; Volume 82, pp. 301–366. [Google Scholar]
- Joint FAO WHO Expert Committee on Food Additives, World Health Organization. Safety evaluation of certain food additives and contaminants: Prepared by the Seventy fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://apps.who.int/iris/bitstream/handle/10665/171781/9789240693982_eng.pdf;jsessionid=79EFFBD803B75293027EDA351F998A18?sequence=3 (accessed on 7 June 2019).
- Burgess, L. General ecology of the fusaria. In Fusarium: Diseases, Biology, and Taxonomy; Nelson, P.E., Toussoun, T.A., Cook, R.J., Eds.; Pennsylvania State University Press: University Park, PA, USA, 1981; pp. 225–235. [Google Scholar]
- Marasas, W. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109, 239–243. [Google Scholar] [PubMed]
- Gullino, M.L.; Minuto, A.; Gilardi, G.; Garibaldi, A. Efficacy of azoxystrobin and other strobilurins against Fusarium wilts of carnation, cyclamen and Paris daisy. Crop. Prot. 2002, 21, 57–61. [Google Scholar] [CrossRef]
- Booth, C. The genus Fusarium. Fusarium; CAB, 1971. Available online: http://www.mycobank.org/BioloMICS.aspx?TableKey=14682616000000061&Rec=744&Fields=All (accessed on 7 June 2019).
- Vujanovic, V.; St-Arnaud, M.; Barabé, D.; Thibeault, G. Viability testing of orchid seed and the promotion of colouration and germination. Ann. Bot. 2000, 86, 79–86. [Google Scholar] [CrossRef]
- Alabouvette, C.; Lemanceau, P.; Steinberg, C. Recent advances in the biological control of Fusarium wilts. Pestic. Sci. 1993, 37, 365–373. [Google Scholar] [CrossRef]
- Louvet, J.; Rouxel, F.; Alabouvette, C. Recherches sur la résistance des sols aux maladies. I. Mise en évidence de la nature microbiologique de la résistance d’un sol au développement de la fusariose vasculaire du melon. Ann. Phytopathol. 1976, 8, 425–436. [Google Scholar]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. 2010, 27, 651–657. [Google Scholar] [CrossRef]
- Ross, P.F.; Rice, L.G.; Osweiler, G.D.; Nelson, P.E.; Richard, J.L.; Wilson, T.M. A review and update of animal toxicoses associated with fumonisin-contaminated feeds and production of fumonisins by Fusarium isolates. Mycopathologia 1992, 117, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.R.; Colvin, B.M.; Greene, J.T.; Newman, L.E.; Cole Jr, J.R. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J. Vet. Diagn. Investig. 1990, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Plattner, R.D.; Riley, R.T.; Meredith, F.I.; Norred, W.P. In vivo effects of fumonisin B 1-producing and fumonisin B 1-nonproducing Fusarium moniliforme isolates are similar: Fumonisins B 2 and B 3 cause hepato-and nephrotoxicity in rats. Mycopathologia 1998, 141, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Ciacci-Zanella, J.R.; Zhang, Y.; Henderson, G.; Dickman, M. Analysis of fumonisin B1-induced apoptosis. Environ. Health Perspect. 2001, 109 (Suppl. 2), 315–320. [Google Scholar] [CrossRef]
- Burgess, K.M.; Renaud, J.B.; McDowell, T.; Sumarah, M.W. Mechanistic insight into the biosynthesis and detoxification of fumonisin mycotoxins. ACS Chem. Biol. 2016, 11, 2618–2625. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Sreelakshmi, L. Formal synthesis of fumonisin B1, a potent sphingolipid biosynthesis inhibitor. Tetrahedron Lett. 2012, 53, 3233–3236. [Google Scholar] [CrossRef]
- Wang, E.; Norred, W.; Bacon, C.; Riley, R.; Merrill, A.H. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar]
- Gurjar, M.K.; Rajendran, V.; Rao, B.V. Chiron approach towards a potent toxin fumonisin B1 backbone: Synthesis of its hexaacetate derivative. Tetrahedron Lett. 1998, 39, 3803–3806. [Google Scholar] [CrossRef]
- Oikawa, H.; Yamawaki, D.; Kagawa, T.; Ichihara, A. Total synthesis of AAL-toxin TA1. Tetrahedron Lett. 1999, 40, 6621–6625. [Google Scholar] [CrossRef]
- Pereira, C.L.; Chen, Y.-H.; McDonald, F.E. Total synthesis of the sphingolipid biosynthesis inhibitor fumonisin B1. J. Am. Chem. Soc. 2009, 131, 6066–6067. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Schmidt-Heydt, M.; Haidukowski, M.; Geisen, R.; Logrieco, A.; Mulè, G. Influence of light on growth, fumonisin biosynthesis and FUM1 gene expression by Fusarium proliferatum. Int. J. Food Microbiol. 2012, 153, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Stępień, Ł.; Waśkiewicz, A.; Wilman, K. Host extract modulates metabolism and fumonisin biosynthesis by the plant-pathogenic fungus Fusarium proliferatum. Int. J. Food Microbiol. 2015, 193, 74–81. [Google Scholar] [CrossRef]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Proctor, R.H.; Brown, D.W.; Plattner, R.D.; Desjardins, A.E. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet. Biol. 2003, 38, 237–249. [Google Scholar] [CrossRef]
- Desjardins, A.; Proctor, R. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef]
- Bojja, R.S.; Cerny, R.L.; Proctor, R.H.; Du, L. Determining the biosynthetic sequence in the early steps of the fumonisin pathway by use of three gene-disruption mutants of Fusarium verticillioides. J. Agric. Food Chem. 2004, 52, 2855–2860. [Google Scholar] [CrossRef]
- López-Errasquín, E.; Vázquez, C.; Jiménez, M.; González-Jaén, M.T. Real-time RT-PCR assay to quantify the expression of FUM1 and FUM19 genes from the fumonisin-producing Fusarium verticillioides. J. Microbiol. Methods 2007, 68, 312–317. [Google Scholar] [CrossRef]
- Seo, J.-A.; Proctor, R.H.; Plattner, R.D. Characterization of four clustered and coregulated genes associated with fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 2001, 34, 155–165. [Google Scholar] [CrossRef]
- Baker, S.E. Aspergillus niger genomics: Past, present and into the future. Med. Mycol. 2006, 44, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Pel, H.J.; De Winde, J.H.; Archer, D.B.; Dyer, P.S.; Hofmann, G.; Schaap, P.J.; Turner, G.; De Vries, R.P.; Albang, R.; Albermann, K. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 2007, 25, 221–231. [Google Scholar] [CrossRef]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Noonim, P.; Mahakarnchanakul, W.; Nielsen, K.F.; Frisvad, J.C.; Samson, R.A. Fumonisin B2 production by Aspergillus niger in Thai coffee beans. Food Addit. Contam. 2009, 26, 94–100. [Google Scholar] [CrossRef]
- Mansson, M.; Klejnstrup, M.L.; Phipps, R.K.; Nielsen, K.F.; Frisvad, J.C.; Gotfredsen, C.H.; Larsen, T.O. Isolation and NMR characterization of fumonisin B2 and a new fumonisin B6 from Aspergillus niger. J. Agric. Food Chem. 2009, 58, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Proctor, R.H.; Butchko, R.A.; Haidukowski, M.; Stea, G.; Logrieco, A.; Moretti, A. Variation in the fumonisin biosynthetic gene cluster in fumonisin-producing and nonproducing black aspergilli. Fungal Genet. Biol. 2014, 73, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Woloshuk, C.P. Functional characterization of fst1 in Fusarium verticillioides during colonization of maize kernels. Mol. Plant Microbe Interact. 2011, 24, 18–24. [Google Scholar] [CrossRef]
- Ridenour, J.B.; Bluhm, B.H. The novel fungal-specific gene FUG1 has a role in pathogenicity and fumonisin biosynthesis in Fusarium verticillioides. Mol. Plant Pathol. 2017, 18, 513–528. [Google Scholar] [CrossRef]
- Niehaus, E.-M.; Münsterkötter, M.; Proctor, R.H.; Brown, D.W.; Sharon, A.; Idan, Y.; Oren-Young, L.; Sieber, C.M.; Novák, O.; Pěnčík, A. Comparative “omics” of the Fusarium fujikuroi species complex highlights differences in genetic potential and metabolite synthesis. Genome Biol. Evol. 2016, 8, 3574–3599. [Google Scholar] [CrossRef]
- Seefelder, W.; Gossmann, M.; Humpf, H.-U. Analysis of fumonisin B1 in Fusarium proliferatum-infected asparagus spears and garlic bulbs from Germany by liquid chromatography− electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2778–2781. [Google Scholar] [CrossRef]
- Park, J.; Kim, E.; Shon, D.; Kim, Y. Natural co-occurrence of aflatoxin B1, fumonisin B1 and ochratoxin A in barley and corn foods from Korea. Food Addit. Contam. 2002, 19, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, L.M.; Vieira, A.P.; Soares, L.M.V. Fumonisin B1 and ochratoxin A in beers made in Brazil. Food Sci. Technol. 2007, 27, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Heperkan, D.; Güler, F.K.; Oktay, H. Mycoflora and natural occurrence of aflatoxin, cyclopiazonic acid, fumonisin and ochratoxin A in dried figs. Food Addit. Contam. Part A 2012, 29, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, T.; Lugoboni, B.; Zironi, E.; Barbarossa, A.; Serraino, A.; Pagliuca, G. Determination of fumonisin B1 in bovine milk by LC–MS/MS. Food Control 2009, 20, 1171–1174. [Google Scholar] [CrossRef]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011, 52, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.; Scriven, F.; Patel, S. Fusarium mycotoxins in the food chain: Maize-based snack foods. World Mycotoxin J. 2009, 2, 441–450. [Google Scholar] [CrossRef]
- Castells, M.; Marín, S.; Sanchis, V.; Ramos, A.J. Distribution of fumonisins and aflatoxins in corn fractions during industrial cornflake processing. Int. J. Food Microbiol. 2008, 123, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.; Silva, L.; Pena, A.; Fernández, M.; Mañes, J. Occurrence of fumonisins B1 and B2 in broa, typical Portuguese maize bread. Int. J. Food Microbiol. 2007, 118, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omuttang, G.Z.; Yaziciogilu, D. Determination of fumonisins B1 and B2 in herbal tea and medicinal plants in Turkey by high-performance liquid chromatography. J. Food Prot. 2004, 67, 1782–1786. [Google Scholar]
- Preis, R.; Vargas, E. A method for determining fumonisin B1 in corn using immunoaffinity column clean-up and thin layer chromatography/densitometry. Food Addit. Contam. 2000, 17, 463–468. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; Sizoo, E.; Vermunt, A.; Notermans, S.; Van Egmond, H. The occurrence of fumonisin B1 in maize-containing foods in the Netherlands. Food Addit. Contam. 1998, 15, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Pittet, A.; Parisod, V.; Schellenberg, M. Occurrence of fumonisins B1 and B2 in corn-based products from the Swiss market. J. Agric. Food Chem. 1992, 40, 1352–1354. [Google Scholar] [CrossRef]
- Omurtag, G.Z. Determination of fumonisin B1 and B2 in corn and corn-based products in Turkey by high-performance liquid chromatography. J. Food Prot. 2001, 64, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Kpodo, K.; Thrane, U.; Hald, B. Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int. J. Food Microbiol. 2000, 61, 147–157. [Google Scholar] [CrossRef]
- Doko, M.B.; Canet, C.; Brown, N.; Sydenham, E.W.; Mpuchane, S.; Siame, B.A. Natural co-occurrence of fumonisins and zearalenone in cereals and cereal-based foods from Eastern and Southern Africa. J. Agric. Food Chem. 1996, 44, 3240–3243. [Google Scholar] [CrossRef]
- Doko, M.B.; Rapior, S.; Visconti, A.; Schjoth, J.E. Incidence and levels of fumonisin contamination in maize genotypes grown in Europe and Africa. J. Agric. Food. Chem. 1995, 43, 429–434. [Google Scholar] [CrossRef]
- De Nijs, M.; Sizoo, E.; Rombouts, F.; Notermans, S.; Van Egmond, H. Fumonisin B1 in maize for food production imported in The Netherlands. Food Addit. Contam. 1998, 15, 389–392. [Google Scholar] [CrossRef]
- Kedera, C.; Plattner, R.; Desjardins, A. Incidence of Fusarium spp. and levels of fumonisin B1 in maize in western Kenya. Appl. Environ. Microbiol. 1999, 65, 41–44. [Google Scholar]
- Medina-Martínez, M.S.; Martínez, A.J. Mold occurrence and aflatoxin B1 and fumonisin B1 determination in corn samples in Venezuela. J. Agric. Food. Chem. 2000, 48, 2833–2836. [Google Scholar] [CrossRef]
- Kim, E.-K.; Shon, D.-H.; Chung, S.-H.; Kim, Y.-B. Survey for fumonisin B1 in Korean corn-based food products. Food Addit. Contam. 2002, 19, 459–464. [Google Scholar] [CrossRef]
- Nayaka, S.C.; Shankar, A.U.; Niranjana, S.; Wulff, E.G.; Mortensen, C.; Prakash, H. Detection and quantification of fumonisins from Fusarium verticillioides in maize grown in southern India. World J. Microbiol. Biotechnol. 2010, 26, 71. [Google Scholar] [CrossRef]
- Shephard, G.S.; Marasas, W.F.; Leggott, N.L.; Yazdanpanah, H.; Rahimian, H.; Safavi, N. Natural occurrence of fumonisins in corn from Iran. J. Agric. Food. Chem. 2000, 48, 1860–1864. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Yamashita, A.; Chokethaworn, N. Occurrence of fumonisins and aflatoxins in corn from Thailand. Food Addit. Contam. 1996, 13, 163–168. [Google Scholar] [CrossRef]
- Ueno, Y.; Aoyama, S.; Sugiura, Y.; Wang, D.; Lee, U.; Hirooka, E.; Hara, S.; Karki, T.; Chen, G.; Yu, S. A limited survey of fumonisins in corn and corn-based products in Asian countries. Mycotoxin Res. 1993, 9, 27–34. [Google Scholar] [CrossRef]
- Ali, N.; Sardjono; Yamashita, A.; Yoshizawa, T. Natural co-occurrence of aflatoxins and Fusavium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 1998, 15, 377–384. [Google Scholar] [CrossRef]
- Palacios, S.A.; Ramirez, M.L.; Cabrera Zalazar, M.; Farnochi, M.C.; Zappacosta, D.; Chiacchiera, S.M.; Reynoso, M.M.; Chulze, S.N.; Torres, A.M. Occurrence of Fusarium spp. and fumonisin in durum wheat grains. J. Agric. Food Chem. 2011, 59, 12264–12269. [Google Scholar] [CrossRef]
- Mendes, G.D.R.L.; Reis, T.A.D.; Corrêa, B.; Badiale-Furlong, E. Mycobiota and occurrence of Fumonisin B1 in wheat harvested in Southern Brazil. Ciênc. Rural. 2015, 45, 1050–1057. [Google Scholar] [CrossRef]
- Roscoe, V.; Lombaert, G.; Huzel, V.; Neumann, G.; Melietio, J.; Kitchen, D.; Kotello, S.; Krakalovich, T.; Trelka, R.; Scott, P. Mycotoxins in breakfast cereals from the Canadian retail market: A 3-year survey. Food Addit. Contam. 2008, 25, 347–355. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Zheng, F.; Chen, J.; Li, W. Fumonisins B1, B2 and B3 in corn products, wheat flour and corn oil marketed in Shandong province of China. Food Addit. Contam. Part B. 2015, 8, 169–174. [Google Scholar] [CrossRef]
- Rubert, J.; Soriano, J.M.; Mañes, J.; Soler, C. Occurrence of fumonisins in organic and conventional cereal-based products commercialized in France, Germany and Spain. Food Chem. Toxicol. 2013, 56, 387–391. [Google Scholar] [CrossRef]
- Chehri, K.; Jahromi, S.T.; Reddy, K.; Abbasi, S.; Salleh, B. Occurrence of Fusarium spp. and fumonisins in stored wheat grains marketed in Iran. Toxins 2010, 2, 2816–2823. [Google Scholar] [CrossRef]
- Cirillo, T.; Ritieni, A.; Galvano, F.; Amodio Cocchieri, R. Natural co-occurrence of deoxynivalenol and fumonisins B1 and B2 in Italian marketed foodstuffs. Food Addit. Contam. 2003, 20, 566–571. [Google Scholar] [CrossRef]
- Kushiro, M.; Zheng, Y.; Nagata, R.; Nakagawa, H.; Nagashima, H. Limited surveillance of fumonisins in brown rice and wheat harvested in Japan. J. Food Prot. 2009, 72, 1327–1331. [Google Scholar] [CrossRef]
- Stanković, S.; Lević, J.; Ivanović, D.; Krnjaja, V.; Stanković, G.; Tančić, S. Fumonisin B1 and its co-occurrence with other fusariotoxins in naturally-contaminated wheat grain. Food Control 2012, 23, 384–388. [Google Scholar] [CrossRef]
- Mashinini, K.; Dutton, M.F. The incidence of fungi and mycotoxins in South Africa wheat and wheat-based products. J. Environ. Sci. Health B. 2006, 41, 285–296. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Serrano, A.; Font, G.; Ruiz, M.; Ferrer, E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012, 135, 423–429. [Google Scholar] [CrossRef]
- Busman, M.; Desjardins, A.; Proctor, R. Analysis of fumonisin contamination and the presence of Fusarium in wheat with kernel black point disease in the United States. Food Addit. Contam. Part A 2012, 29, 1092–1100. [Google Scholar] [CrossRef]
- Gamanya, R.; Sibanda, L. Survey of Fusarium moniliforme (F. verticillioides) and production of fumonisin B1 in cereal grains and oilseeds in Zimbabwe. Int. J. Food Microbiol. 2001, 71, 145–149. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Santiago, R.; Ramos, A.J.; Marín, S.; Reid, L.M.; Butrón, A. Environmental factors related to fungal infection and fumonisin accumulation during the development and drying of white maize kernels. Int. J. Food Microbiol. 2013, 164, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irzykowska, L.; Bocianowski, J.; Waśkiewicz, A.; Weber, Z.; Karolewski, Z.; Goliński, P.; Kostecki, M.; Irzykowski, W. Genetic variation of Fusarium oxysporum isolates forming fumonisin B 1 and moniliformin. J. Appl. Genet. 2012, 53, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Stępień, Ł.; Wilman, K.; Kachlicki, P. Diversity of pea-associated F. proliferatum and F. verticillioides populations revealed by FUM1 sequence analysis and fumonisin biosynthesis. Toxins 2013, 5, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Monge, M.P.; Palacios, S.A.; Chiacchiera, S.M.; Torres, A.M.; Farnochi, M.C.; Ramirez, M.L. Fumonisin occurrence in naturally contaminated wheat grain harvested in Argentina. Food Control. 2014, 37, 56–61. [Google Scholar] [CrossRef]
- Wong, J.; Jeffries, P. Diversity of pathogenic Fusarium populations associated with asparagus roots in decline soils in Spain and the UK. Plant Physiol. 2006, 55, 331–342. [Google Scholar] [CrossRef]
- Karbancıoglu-Güler, F.; Heperkan, D. Natural occurrence of fumonisin B1 in dried figs as an unexpected hazard. Food Chem. Toxicol. 2009, 47, 289–292. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.C.C.; Lala, B.; Carniato, C.; Schamberd, C.R.; Nascimento, C.S.; Braccini, G.L.; Porto, C.; Roldi, G.; Tanamati, F.; Gasparino, E. Fumonisin affects performance and modulates the gene expression of IGF-1 and GHR in Nile tilapia fingerlings and juveniles. Aquaculture 2019, 507, 233–237. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gutema, T.; Munimbazi, C.; Bullerman, L.B. Occurrence of fumonisins and moniliformin in corn and corn-based food products of US origin. J. Food Prot. 2000, 63, 1732–1737. [Google Scholar] [CrossRef]
- Martins, F.A.; Ferreira, F.M.D.; Ferreira, F.D.; Bando, É.; Nerilo, S.B.; Hirooka, E.Y.; Machinski Jr, M. Daily intake estimates of fumonisins in corn-based food products in the population of Parana, Brazil. Food Control. 2012, 26, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.-G.; Phat, C.; Kim, D.-H.; Lee, C. Occurrence of Fusarium mycotoxin fumonisin B 1 and B 2 in animal feeds in Korea. Mycotoxin Res. 2013, 29, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.F.; Krska, R.; Sulyok, M. Occurrence of Ochratoxins, Fumonisin B2, Aflatoxins (B1 and B2), and Other Secondary Fungal Metabolites in Dried Date Palm Fruits from Egypt: A Mini-Survey. J. Food Sci. 2018, 83, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Rosa Junior, O.F.; Dalcin, M.S.; Nascimento, V.L.; Haesbaert, F.M.; Ferreira, T.P.S.; Fidelis, R.R.; Sarmento, R.A.; Aguiar, R.W.S.; Oliveira, E.E.; Santos, G.R. Fumonisin Production by Fusarium verticillioides in Maize Genotypes Cultivated in Different Environments. Toxins 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S. Occurrence of aflatoxins, ochratoxin A, and fumonisins in retail foods in Japan. J. Food Prot. 2006, 69, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- García-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Analysis of mycotoxins in coffee and risk assessment in Spanish adolescents and adults. Food Chem. Toxicol. 2015, 86, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.C. Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to ergot as undesirable substance in animal feed: Question No EFSA-Q-2003-38. EFSA J. 2005. [Google Scholar] [CrossRef]
- Norred, W.; Plattner, R.; Vesonder, R.; Bacon, C.; Voss, K. Effects of selected secondary metabolites of Fusarium moniliforme on unscheduled synthesis of DNA by rat primary hepatocytes. Food Chem. Toxicol. 1992, 30, 233–237. [Google Scholar] [CrossRef]
- Voss, K.; Smith, G.; Haschek, W. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci.Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill Jr, A.H.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas–Mexico border. Environ. Health Perspect. 2005, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, W.C.; Kriek, N.; Marasas, W.; Thiel, P. Toxicity and carcinogenicity of the Fusanum monilzforine metabolite, fumonisin B1, in rats. Carcinogenesis 1991, 12, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Marasas, D.; WFO, J. Fusarium moniliforme contamination of maize in oesophageal cancer areas in Transkei. S. Afr. Med. J. 1988, 74, 110–114. [Google Scholar] [PubMed]
- Franceschi, S.; Bidoli, E.; Barón, A.E.; La Vecchia, C. Maize and risk of cancers of the oral cavity, pharynx, and esophagus in northeastern Italy. J. Natl. Cancer Inst. 1990, 82, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Dragan, Y.P.; Bidlack, W.R.; Cohen, S.M.; Goldsworthy, T.L.; Hard, G.C.; Howard, P.C.; Riley, R.T.; Voss, K.A. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: Fumonisin B1 as an example. Toxicol. Sci. 2001, 61, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Rumora, L.; Kovačić, S.; Rozgaj, R.; Čepelak, I.; Pepeljnjak, S.; Grubišić, T.Ž. Cytotoxic and genotoxic effects of fumonisin B 1 on rabbit kidney RK13 cell line. Arch. Toxicol. 2002, 76, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.-S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.M.; Roshandel, G.; Roudbarmohammadi, S.; Roudbary, M.; Sohanaki, H.; Ghiasian, S.A.; Taherkhani, A.; Semnani, S.; Aghasi, M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac. J. Cancer Prev. 2012, 13, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Chulze, S.; Mallmann, C.; Visconti, A.; De Girolamo, A.; Rojo, F.; Torres, A. Comparison of urinary sphingolipids in human populations with high and low maize consumption as a possible biomarker of fumonisin dietary exposure. Food Addit. Contam. 2004, 21, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Haliburton, J.C.; Buck, W.B. Equine leucoencephalomalacia: An historical review. In Diagnosis of Mycotoxicoses; Springer: Berlin/Heidelberg, Germany, 1986; pp. 75–79. [Google Scholar]
- Kriek, N.; Kellerman, T.S.; Marasas, W.F.O. A comparative study of the toxicity of Fusarium verticillioides (=F. moniliforme) to horses, primates, pigs, sheep and rats. Onderstepoort J. Vet. Res. 1981, 48, 129–131. [Google Scholar]
- Marasas, W.F.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.; Merrill, A.H.; Stevens, V.L.; Sullards, M.C.; Wang, E.; Wang, P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology 2002, 66, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.-S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Liverpool-Tasie, L.; Turna, N.S.; Ademola, O.; Obadina, A.; Wu, F. The occurrence and co-occurrence of aflatoxin and fumonisin along the maize value chain in southwest Nigeria. Food Chem. Toxicol. 2019, 129, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Mwalwayo, D.S.; Thole, B. Prevalence of aflatoxin and fumonisins (B1+ B2) in maize consumed in rural Malawi. Toxicol. Rep. 2016, 3, 173–179. [Google Scholar] [CrossRef]
- Polychronaki, N.; West, R.M.; Turner, P.C.; Amra, H.; Abdel-Wahhab, M.; Mykkänen, H.; El-Nezami, H. A longitudinal assessment of aflatoxin M1 excretion in breast milk of selected Egyptian mothers. Food Chem. Toxicol. 2007, 45, 1210–1215. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Arefhosseini, S.; Jabbari, M.V. Determination of Aflatoxin M 1 in Breast Milk Samples in Tabriz–Iran. Matern. Child Health J. 2010, 14, 141–145. [Google Scholar] [CrossRef]
- Magoha, H.; Kimanya, M.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Association between aflatoxin M1 exposure through breast milk and growth impairment in infants from Northern Tanzania. Matern. World Mycotoxin J. 2014, 7, 277–284. [Google Scholar] [CrossRef]
- Kimanya, M.E.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010, 54, 1659–1667. [Google Scholar] [CrossRef]
- Shirima, C.P.; Kimanya, M.E.; Routledge, M.N.; Srey, C.; Kinabo, J.L.; Humpf, H.-U.; Wild, C.P.; Tu, Y.-K.; Gong, Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect. 2014, 123, 173–178. [Google Scholar] [CrossRef]
- Chen, C.; Mitchell, N.J.; Gratz, J.; Houpt, E.R.; Gong, Y.; Egner, P.A.; Groopman, J.D.; Riley, R.T.; Showker, J.L.; Svensen, E. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environ. Int. 2018, 115, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F. Feeding and Nutrition of Infants and Young Children: Guidelines for the WHO European Region, with Emphasis on the Former Soviet Countries; WHO Regional Office Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Humpf, H.U.; Voss, K.A. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 2004, 48, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, C.; Cirlini, M.; Galaverna, G.; Dall’Asta, C. Masked fumonisins in processed food: Co-occurrence of hidden and bound forms and their stability under digestive conditions. World Mycotoxin J. 2012, 5, 325–334. [Google Scholar] [CrossRef]
- Seefelder, W.; Hartl, M.; Humpf, H.-U. Determination of N-(carboxymethyl) fumonisin B1 in corn products by liquid chromatography/electrospray ionization–mass spectrometry. J. Agric. Food Chem. 2001, 49, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Poling, S.M.; Plattner, R.D.; Weisleder, D. N-(1-Deoxy-D-fructos-1-yl) fumonisin B1, the initial reaction product of fumonisin B1 and D-glucose. J. Agric. Food. Chem. 2002, 50, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.S.; Meredith, F.I.; Voss, K.A. Control of fumonisin: Effects of processing. Environ. Health Perspect. 2001, 109, 333–336. [Google Scholar] [PubMed]
- Scudamore, K.; Patel, S. Fusarium mycotoxins in milling streams from the commercial milling of maize imported to the UK, and relevance to current legislation. Food Addit. Contam. 2009, 26, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Shephard, G.; Louw, W.; Rheeder, J.; Gelderblom, W. The mycotoxin distribution in maize milling fractions under experimental conditions. Int. J. Food Microbiol. 2013, 165, 57–64. [Google Scholar] [CrossRef]
- Scudamore, K. Fate of Fusarium mycotoxins in the cereal industry: Recent UK studies. World Mycotoxin J. 2008, 1, 315–323. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the Processes of Nixtamalization and Tortilla Production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Ryu, D.; Jackson, L.S. Stability of fumonisins in food processing. In Mycotoxins and Food Safety; Springer: Berlin/Heidelberg, Germany, 2002; pp. 195–204. [Google Scholar]
- Magan, N. Fungi in extreme environments. Mycota 2007, 4, 85–103. [Google Scholar]
- Jurado, M.; Vázquez, C.; Callejas, C.; González-Jaén, M. Occurrence and variability of mycotoxigenic Fusarium species associated to wheat and maize in the South West of Spain. Mycotoxin Res. 2006, 22, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Aliakbari, F.; Mirabolfathy, M.; Emami, M.; Mazhar, S.F.; Karami-Osboo, R. Natural occurrence of Fusarium species in maize kernels at Gholestan province in northern Iran. Asian J. Plant Sci. 2007, 8, 1276–1281. [Google Scholar]
- Cavaglieri, L.; Keller, K.; Pereyra, C.; Pereyra, M.G.; Alonso, V.; Rojo, F.; Dalcero, A.; Rosa, C. Fungi and natural incidence of selected mycotoxins in barley rootlets. J. Stored Prod. Res. 2009, 45, 147–150. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Mateo, E.; González-Jaén, M.; Jiménez, M.; Vázquez, C.; Patiño, B. Contamination of barley seeds with Fusarium species and their toxins in Spain: An integrated approach. Food Addit. Contam. Part A 2013, 30, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Maiorano, A.; Reyneri, A.; Magni, A.; Ramponi, C. A decision tool for evaluating the agronomic risk of exposure to fumonisins of different maize crop management systems in Italy. Agric. Syst. 2009, 102, 17–23. [Google Scholar] [CrossRef]
- Cao, A.; Santiago, R.; Ramos, A.J.; Souto, X.C.; Aguín, O.; Malvar, R.A.; Butrón, A. Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in northwestern Spain. Int. J. Food Microbiol. 2014, 177, 63–71. [Google Scholar] [CrossRef]
- Czembor, E.; Waśkiewicz, A.; Piechota, U.; Puchta, M.; Czembor, J.H.; Stepien, L. Differences in ear rot resistance and Fusarium verticillioides-produced fumonisin contamination between Polish currently and historically used maize inbred lines. Front. Microbiol. 2019, 10, 449. [Google Scholar] [CrossRef]
- Cendoya, E.; del Pilar Monge, M.; Chiacchiera, S.M.; Farnochi, M.C.; Ramirez, M.L. Influence of water activity and temperature on growth and fumonisin production by Fusarium proliferatum strains on irradiated wheat grains. Int. J. Food Microbiol. 2018, 266, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kohut, G.; Ádám, A.L.; Fazekas, B.; Hornok, L. N-starvation stress induced FUM gene expression and fumonisin production is mediated via the HOG-type MAPK pathway in Fusarium proliferatum. Int. J. Food Microbiol. 2009, 130, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gong, L.; Wang, Y.; Chen, F.; Gupta, V.K.; Jian, Q.; Duan, X.; Jiang, Y. Proteomics analysis of Fusarium proliferatum under various initial pH during fumonisin production. J. Proteom. 2017, 164, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Sullivan, T.; Chirtel, S. Factors affecting the growth of Fusarium proliferatum and the production of fumonisin B 1: Oxygen and pH. J. Ind. Microbiol. Biotechnol. 1997, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, J.G. (Eds.) Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. Simple and efficient methodology to determine mycotoxins in cereal syrups. Food Chem. 2015, 177, 274–279. [Google Scholar] [CrossRef]
- Bolechová, M.; Benešová, K.; Běláková, S.; Čáslavský, J.; Pospíchalová, M.; Mikulíková, R. Determination of seventeen mycotoxins in barley and malt in the Czech Republic. Food Control. 2015, 47, 108–113. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Ngemela, A.F.; Jensen, L.B.; De Medeiros, L.S.; Rasmussen, P.H. UHPLC-MS/MS determination of ochratoxin A and fumonisins in coffee using QuEChERS extraction combined with mixed-mode SPE purification. J. Agric. Food Chem. 2015, 63, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, C.; Guo, J.; Yuan, Y.; Wang, J.; Liu, L.; Yue, T. Development and Application of a Method for the Analysis of 9 Mycotoxins in Maize by HPLC-MS/MS. J. Food Sci. 2013, 78, M1752–M1756. [Google Scholar] [CrossRef] [PubMed]
- Ediage, E.N.; Van Poucke, C.; De Saeger, S. A multi-analyte LC–MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 177, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-Y.; Choe, B.-C.; Choi, E.-J.; Jeong, H.-J.; Hwang, Y.-S.; Shin, G.-Y.; Kim, J.-H. Survey of mycotoxins in commonly consumed Korean grain products using an LC-MS/MS multimycotoxin method in combination with immunoaffinity clean-up. Food Sci. Biotechnol. 2015, 24, 1193–1199. [Google Scholar] [CrossRef]
- Bryła, M.; Roszko, M.; Szymczyk, K.; Jędrzejczak, R.; Obiedziński, M.W. Fumonisins and their masked forms in maize products. Food Control 2016, 59, 619–627. [Google Scholar] [CrossRef]
- Beltrán, E.; Ibáñez, M.; Portolés, T.; Ripollés, C.; Sancho, J.V.; Yusà, V.; Marín, S.; Hernández, F. Development of sensitive and rapid analytical methodology for food analysis of 18 mycotoxins included in a total diet study. Anal. Chim. Acta 2013, 783, 39–48. [Google Scholar] [CrossRef] [PubMed]
- García-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Development of a new method for the simultaneous determination of 21 mycotoxins in coffee beverages by liquid chromatography tandem mass spectrometry. Food Res. Int. 2015, 72, 247–255. [Google Scholar] [CrossRef]
- Rubert, J.; Dzuman, Z.; Vaclavikova, M.; Zachariasova, M.; Soler, C.; Hajslova, J. Analysis of mycotoxins in barley using ultra high liquid chromatography high resolution mass spectrometry: Comparison of efficiency and efficacy of different extraction procedures. Talanta 2012, 99, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Moltó, J.-C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control. 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. A new approach in sample treatment combined with UHPLC-MS/MS for the determination of multiclass mycotoxins in edible nuts and seeds. Talanta 2013, 115, 61–67. [Google Scholar] [CrossRef]
- Ye, H.; Lai, X.; Liu, C. Determination of Fumonisin B 1 and B 2 in Corn Using Matrix-Phase Dispersion Coupled to High Performance Liquid Chromatography. Asian J. Chem. 2013, 25, 6807–6810. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Rodrigues, M.I.; Rossi, E.A.; de Sylos, C.M. Optimisation of a sample preparation method for the determination of fumonisin B1 in rice. Food Chem. 2014, 158, 270–277. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Rossi, E.A.; de Sylos, C.M. In-house method validation, estimating measurement uncertainty and the occurrence of fumonisin B1 in samples of Brazilian commercial rice. Food Control 2016, 59, 439–446. [Google Scholar] [CrossRef]
- Zhang, K.; Wong, J.W.; Hayward, D.G.; Vaclavikova, M.; Liao, C.-D.; Trucksess, M.W. Determination of mycotoxins in milk-based products and infant formula using stable isotope dilution assay and liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 6265–6273. [Google Scholar] [CrossRef]
- Azaiez, I.; Giusti, F.; Sagratini, G.; Mañes, J.; Fernández-Franzón, M. Multi-mycotoxins analysis in dried fruit by LC/MS/MS and a modified QuEChERS procedure. Food Anal. Methods 2014, 7, 935–945. [Google Scholar] [CrossRef]
- Liao, C.-D.; Wong, J.W.; Zhang, K.; Yang, P.; Wittenberg, J.B.; Trucksess, M.W.; Hayward, D.G.; Lee, N.S.; Chang, J.S. Multi-mycotoxin analysis of finished grain and nut products using ultrahigh-performance liquid chromatography and positive electrospray ionization–quadrupole orbital ion trap high-resolution mass spectrometry. J. Agric. Food Chem. 2015, 63, 8314–8332. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Rodrigues, P.; Freitas-Silva, O.; Abrunhosa, L.; Venâncio, A. HPLC method for simultaneous detection of aflatoxins and cyclopiazonic acid. World Mycotoxin J. 2010, 3, 225–231. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; He, Q.-H.; He, Z.-Y.; Xiong, Z.-P. Application of mimotope peptides of fumonisin B1 in Peptide ELISA. J. Agric. Food Chem. 2013, 61, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Wang, C.; Han, Z.; Wu, A.; Zhang, D.; Shi, J. Determination of deoxynivalenol (DON) and its derivatives: Current status of analytical methods. Food Control. 2013, 34, 138–148. [Google Scholar] [CrossRef]
- Lee, N.A.; Wang, S.; Allan, R.D.; Kennedy, I.R. A rapid aflatoxin B1 ELISA: Development and validation with reduced matrix effects for peanuts, corn, pistachio, and soybeans. J. Agric. Food Chem. 2004, 52, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, D.; Adkar-Purushothama, C.R.; Yanjarappa, S.M. Multiplex PCR for the early detection of fumonisin producing Fusarium verticillioides. Food Biosci. 2016, 13, 84–88. [Google Scholar] [CrossRef]
- Sreenivasa, M.; Dass, R.S.; Raj, A.C.; Janardhana, G. Molecular detection of fumonisin producing Fusarium species of freshly harvested maize kernels using polymerase chain reaction (PCR). Taiwania 2006, 51, 251–257. [Google Scholar]
- Bintvihok, A.; Treebonmuang, S.; Srisakwattana, K.; Nuanchun, W.; Patthanachai, K.; Usawang, S. A rapid and sensitive detection of aflatoxin-producing fungus using an optimized polymerase chain reaction (PCR). Toxicol. Res. 2016, 32, 81–87. [Google Scholar] [CrossRef]
- Niessen, L. PCR-based diagnosis and quantification of mycotoxin-producing fungi. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 54, pp. 81–138. [Google Scholar]
- Omori, A.M.; Ono, E.Y.S.; Bordini, J.G.; Hirozawa, M.T.; Fungaro, M.H.P.; Ono, M.A. Detection of Fusarium verticillioides by PCR-ELISA based on FUM21 gene. Food Microbiol. 2018, 73, 160–167. [Google Scholar] [CrossRef]
- Tang, X.; Li, P.; Zhang, Z.; Zhang, Q.; Guo, J.; Zhang, W. An ultrasensitive gray-imaging-based quantitative immunochromatographic detection method for fumonisin B1 in agricultural products. Food Control. 2017, 80, 333–340. [Google Scholar] [CrossRef]
- Del Fiore, A.; Reverberi, M.; Ricelli, A.; Pinzari, F.; Serranti, S.; Fabbri, A.; Bonifazi, G.; Fanelli, C. Early detection of toxigenic fungi on maize by hyperspectral imaging analysis. Int. J. Food Microbiol. 2010, 144, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kimuli, D.; Wang, W.; Lawrence, K.C.; Yoon, S.-C.; Ni, X.; Heitschmidt, G.W. Utilisation of visible/near-infrared hyperspectral images to classify aflatoxin B1 contaminated maize kernels. Biosyst. Eng. 2018, 166, 150–160. [Google Scholar] [CrossRef]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Seenivasan, R.; Wang, Y.-C.; Yu, J.-H.; Gunasekaran, S. An electrochemical immunosensor for rapid and sensitive detection of mycotoxins fumonisin B1 and deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Mao, L.; Ji, K.; Yao, L.; Xue, X.; Wen, W.; Zhang, X.; Wang, S. Molecularly imprinted photoelectrochemical sensor for fumonisin B1 based on GO-CdS heterojunction. Biosens. Bioelectron. 2019, 127, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Young, J.C.; Trenholm, H.L. Fusarium toxins in field corn. I. Time course of fungal growth and production of deoxynivalenol and other mycotoxins. Can. J. Bot. 1983, 61, 3080–3087. [Google Scholar] [CrossRef]
- Scott, P.M.; Nelson, K.; Kanhere, S.R.; Karpinski, K.F.; Hayward, S.; Neish, G.A.; Teich, A.H. Decline in deoxynivalenol (vomitoxin) concentrations in 1983 Ontario winter wheat before harvest. Appl. Environ. Microbiol. 1984, 48, 884–886. [Google Scholar]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. J. Vet. Med. B 1990, 37, 236–240. [Google Scholar] [CrossRef]
- Berthiller, F.; Maragos, C.M.; Dall’Asta, C. Introduction to masked mycotoxins. In Masked Mycotoxins in Food: Formation, Occurrence and Toxicological Relevance; Royal Society of Chemistry, RSC Publishing: Cambridge, UK, 2015; pp. 1–13. [Google Scholar] [CrossRef]
- Dellafiora, L.; Dall’Asta, C. Masked mycotoxins: An emerging issue that makes renegotiable what is ordinary. Food Chem. 2016, 213, 534–535. [Google Scholar] [CrossRef]
- Tran, S.; Smith, T. Determination of optimal conditions for hydrolysis of conjugated deoxynivalenol in corn and wheat with trifluoromethanesulfonic acid. Anim. Feed Sci. Technol. 2011, 163, 84–92. [Google Scholar] [CrossRef]
- McCormick, S.P.; Kato, T.; Maragos, C.M.; Busman, M.; Lattanzio, V.M.; Galaverna, G.; Dall-Asta, C.; Crich, D.; Price, N.P.; Kurtzman, C.P. Anomericity of T-2 toxin-glucoside: Masked mycotoxin in cereal crops. J. Agric. Food Chem. 2015, 63, 731–738. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Binder, S.; Schwartz-Zimmermann, H.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Duvick, J.; Rood, T.; Maddox, J.; Gilliam, J. Molecular Genetics of Host-Specific Toxins in Plant Disease; Springer: Berlin/Heidelberg, Germany, 1998; pp. 369–381. [Google Scholar]
- Blackwell, B.A.; Gilliam, J.T.; Savard, M.E.; David Miller, J.; Duvick, J.P. Oxidative deamination of hydrolyzed fumonisin B1 (AP1) by cultures of Exophiala spinifera. Nat. Toxins 1999, 7, 31–38. [Google Scholar] [CrossRef]
- Duvick, J.; Maddox, J.; Gilliam, J. Compositions and methods for fumonisin detoxification. U.S. Patent US6482621B1, 19 November 2002. [Google Scholar]
- Gu, M.J.; Han, S.E.; Hwang, K.; Mayer, E.; Reisinger, N.; Schatzmayr, D.; Park, B.-C.; Han, S.H.; Yun, C.-H. Hydrolyzed fumonisin B1 induces less inflammatory responses than fumonisin B1 in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol. Lett. 2019, 305, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, R.; Nazzi, F.; Locci, R.; Firrao, G. Degradation of fumonisin B1 by a bacterial strain isolated from soil. Biodegradation 2006, 17, 31–38. [Google Scholar] [CrossRef]
- Täubel, M. Isolierung und Charakterisierung von Mikroorganismen zur biologischen Inaktivierung von Fumonisinen. Doctoral thesis, University of Natural Resources and Applied Life Sciences, Vienna, Austria, 2005. [Google Scholar]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Vekiru, E.; Krska, R.; Schatzmayr, G.; Moll, W.-D.; Grabherr, R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010, 145, 120–129. [Google Scholar] [CrossRef]
- Velluti, A.; Sanchis, V.; Ramos, A.; Egido, J.; Marın, S. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int. J. Food Microbiol. 2003, 89, 145–154. [Google Scholar] [CrossRef]
- Patil, J.R.; Jayaprakasha, G.; Murthy, K.C.; Tichy, S.E.; Chetti, M.B.; Patil, B.S. Apoptosis-mediated proliferation inhibition of human colon cancer cells by volatile principles of Citrus aurantifolia. Food Chem. 2009, 114, 1351–1358. [Google Scholar] [CrossRef]
- Driehuis, F.; Te Giffel, M.; Van Egmond, H.; Fremy, J.; Blüthgen, A. Feed-associated mycotoxins in the dairy chain: Occurrence and control. Fil-Idf Bull. Fed. Int. Lait. Int. Dairy Fed. Brussels – Belgium 2010, 444, 2. [Google Scholar]
- Okabe, I.; Hiraoka, H.; Miki, K. Influence of harvest time on fumonisin contamination of forage maize for whole-crop silage. Mycoscience 2015, 56, 470–475. [Google Scholar] [CrossRef]
- Bush, B.; Carson, M.; Cubeta, M.; Hagler, W.; Payne, G. Infection and fumonisin production by Fusarium verticillioides in developing maize kernels. Phytopathology 2004, 94, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Harrison, J.; Hunt, C.; Shinners, K.; Doggett, C.; Sapienza, D. Nutritive value of corn silage as affected by maturity and mechanical processing: A contemporary review. J. Dairy Sci. 1999, 82, 2813–2825. [Google Scholar] [CrossRef]
- Shephard, G.S.; Burger, H.-M.; Rheeder, J.P.; Alberts, J.F.; Gelderblom, W.C. The effectiveness of regulatory maximum levels for fumonisin mycotoxins in commercial and subsistence maize crops in South Africa. Food Control 2019, 97, 77–80. [Google Scholar] [CrossRef]
- Miller, J.D. Factors that affect the occurrence of fumonisin. Environ. Health Perspect. 2001, 109, 321–324. [Google Scholar] [PubMed]
- van der Westhuizen, L.; Shephard, G.S.; Burger, H.M.; Rheeder, J.P.; Gelderblom, W.C.; Wild, C.P.; Gong, Y.Y. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol. Biomark. Prev. 2011, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Falcão, V.C.A.; Ono, M.A.; de Ávila Miguel, T.; Vizoni, E.; Hirooka, E.Y.; Ono, E.Y.S. Fusarium verticillioides: Evaluation of fumonisin production and effect of fungicides on in vitro inhibition of mycelial growth. Mycopathologia 2011, 171, 77–84. [Google Scholar] [CrossRef]
- Miguel, T.Á.; Bordini, J.G.; Saito, G.H.; Andrade, C.G.J.; Ono, M.A.; Hirooka, E.Y.; Vizoni, É.; Ono, E. Effect of fungicide on Fusarium verticillioides mycelial morphology and fumonisin B1 production. Braz. J. Microbiol. 2015, 46, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In Vitro and in Field Response of Different Fungicides against Aspergillus flavus and Fusarium Species Causing Ear Rot Disease of Maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Geisen, R. Gene expression as an indication for ochratoxin A biosynthesis in Penicillium nordicum. Mycotoxin Res. 2007, 23, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Magan, N.; Geisen, R. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol. Lett. 2008, 284, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, P.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum. FEMS Microbiol. Ecol. 2010, 73, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. Part A 2012, 29, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Buerstmayr, H.; Krska, R.; Schuhmacher, R.; Grausgruber, H.; Ruckenbauer, P. The effect of inoculation treatment and long-term application of moisture on Fusarium head blight symptoms and deoxynivalenol contamination in wheat grains. Eur. J. Plant Pathol. 2004, 110, 299–308. [Google Scholar] [CrossRef]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakajima, T.; Tomimura, K.; Suzuki, F.; Arai, M.; Miyasaka, A. Effect of the timing of fungicide application on Fusarium head blight and mycotoxin contamination in wheat. Plant Dis. 2012, 96, 845–851. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Alberts, J.F.; Van Zyl, W.H.; Gelderblom, W.C. Biologically based methods for control of fumonisin-producing Fusarium species and reduction of the fumonisins. Front. Microbiol. 2016, 7, 548. [Google Scholar] [CrossRef]
- Jacela, J.Y.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Renter, D.G.; Dritz, S.S. Feed additives for swine: Fact sheets–flavors and mold inhibitors, mycotoxin binders, and antioxidants. Kansas Agric. Exp. Station Res. Rep. 2010, 10, 27–32. [Google Scholar] [CrossRef]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D. Biological strategies to counteract the effects of mycotoxins. J. Food Prot. 2009, 72, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, L.W. Evaluation of mycotoxin binders. In Proceedings of the 4th Mid-Atlantic Nutrition Conference, Timonium, Maryland, 2006; pp. 132–143. [Google Scholar]
- De Mil, T.; Devreese, M.; De Baere, S.; Van Ranst, E.; Eeckhout, M.; De Backer, P.; Croubels, S. Characterization of 27 mycotoxin binders and the relation with in vitro zearalenone adsorption at a single concentration. Toxins 2015, 7, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Nazarizadeh, H.; Pourreza, J. Evaluation of three mycotoxin binders to prevent the adverse effects of aflatoxin B1 in growing broilers. J. Appl. Anim. Res. 2019, 47, 135–139. [Google Scholar] [CrossRef] [Green Version]
| Country | Food Matrix | FB1 (Range, µg/kg) | FB2 (Range, µg/kg) | Detection Technique | Reference |
|---|---|---|---|---|---|
| UK | Corn | 200–6000 | - | TLC | [75] |
| The Netherlands | Corn flour | 40–90 | - | HPLC | [76] |
| Switzerland | Corn grits | 0–790 | 0–160 | HPLC | [77] |
| Turkey | Cornmeal | 250–2660 | 550 | HPLC | [78] |
| Ghana | Corn | 11–1655 | 10–770 | HPLC | [79] |
| Malawi | Corn | 20–115 | 30 | HPLC | [80] |
| Zambia | Corn inbred lines | 20–1420 | 10–290 | HPLC | [81] |
| Bahrain | Corn kernel | 25 | - | HPLC | [82] |
| Kenya | Corn kernel | 110–120 | - | HPLC | [83] |
| Venezuela | Yellow corn | 40–15,050 | - | HPLC | [84] |
| Korea | Corn for popping | 23–1210 | - | direct competitive (dcELISA) and HPLC | [85] |
| India | Corn seed samples | 133 to 1617 | - | HPLC | [86] |
| Iran | Corn | 10–3980 | <10–1180 | HPLC | [87] |
| Thailand | Corn | 63–18,800 | 50–1400 | HPLC | [88] |
| Nepal | Corn kernels | 50–4600 | 100–5500 | HPLC | [89] |
| Indonesia | Corn kernels | 51–2440 | <376 | HPLC and GCMS | [90] |
| Argentina | Durum wheat | 10.50–987.20 | 15–258.50 | HPLC-MS/MS | [91] |
| Brazil | Wheat | 958–4906 | - | HPLC-FL | [92] |
| Canada | Wheat | - | - | HPLC | [93] |
| Central Europe | Wheat/wheat bran | - | - | ELISA | [94] |
| China | Wheat flour | 0.30–34.60 | - | UPLC-MS-MS | [95] |
| France | Organic Oat, rye and wheat flakes with maple syrup | 75.70–98.10 | 62.10–81.10 | HPLC-MS/MS | [96] |
| Germany | Organic wheat flakes | 20.20–59.80 | 25.40–41.80 | HPLC-MS/MS | [96] |
| Iran | Stored wheat samples | 15–155 | 12–86 | HPLC | [97] |
| Italy | Cereals, whole meal flours | 10–2870 | 10–420 | LC-MS | [98] |
| Japan | Wheat | >10 | - | LC-ESI-MSMS | [99] |
| Serbia | Wheat | 750–5400 | - | ELISA | [100] |
| South Africa | Wheat and wheat products | 1000–30,000 | - | TLC, HPLC, Ms/MS | [101] |
| South America | Wheat/wheat bran | - | - | ELISA-HPLC | [94] |
| South-East Asia | Wheat/wheat bran | - | - | ELISA-HPLC | [94] |
| Southern Europe | Wheat/wheat bran | - | - | ELISA-HPLC | [94] |
| Spain | Wheat Gofio | 787.50–1001.40 | 645.20–952.10 | HPLC-MS/MS | [96] |
| Syria | Durum wheat | 5–6 | 12 | HPLC-MS/MS | [102] |
| Tunisia | Wheat-based products | 88.33–184 | 121–158 | LC-MS/MS | [103] |
| United States | Wheat | 5–2210 | 2-249 | LC-MS | [104] |
| Zimbabwe | Wheat | 2500–6000 | - | HPLC | [105] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. https://doi.org/10.3390/toxins11060328
Kamle M, Mahato DK, Devi S, Lee KE, Kang SG, Kumar P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins. 2019; 11(6):328. https://doi.org/10.3390/toxins11060328
Chicago/Turabian StyleKamle, Madhu, Dipendra K. Mahato, Sheetal Devi, Kyung Eun Lee, Sang G. Kang, and Pradeep Kumar. 2019. "Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies" Toxins 11, no. 6: 328. https://doi.org/10.3390/toxins11060328
APA StyleKamle, M., Mahato, D. K., Devi, S., Lee, K. E., Kang, S. G., & Kumar, P. (2019). Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins, 11(6), 328. https://doi.org/10.3390/toxins11060328