Defensive Venoms: Is Pain Sufficient for Predator Deterrence?
Abstract
:1. Introduction
2. Results
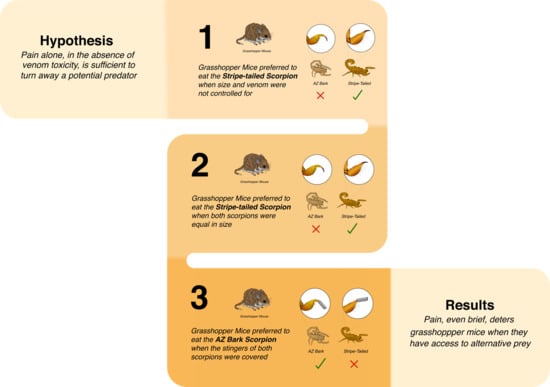
2.1. Mice Prefer to Pursue, Attack, and Consume Less-Painful Scorpions
2.1.1. Experiment 1
2.1.2. Experiment 2
2.1.3. Experiment 3
2.2. Mice Are More Cautious When Pursuing Scorpions with Painful Stings
2.3. Painful Stings Inhibit but Do Not Prevent Mice from Making Subsequent Attacks
2.4. Grasshopper Mice Appear Not to Anticipate the Location of Painful Prey
2.5. Handling Times Increase with Sting Painfulness
2.6. Arizona Bark Scorions Have a Higher Energy Content
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Care and Use Protocols
5.2. Study Subjects
5.2.1. Mice
5.2.2. Scorpions
5.3. Single-Cup Training Trials
5.4. Double-Cup Preference Tests
5.5. Behavrioral Analyses
5.6. Scorpion Energy Content
5.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Koludarov, I.; Jackson, T.N.W.; Holford, M.; Terrat, Y.; Casewell, N.R.; Undheim, E.A.B.; Vetter, I.; Ali, S.A.; Low, D.H.W.; et al. Seeing the woods for the trees: Understanding venom evolution as a guide for biodiscovery. In Venoms to Drugs: Venom as a Source for the Development of Human Therapeutics; King, G.F., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 1–36. [Google Scholar] [CrossRef]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.K.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: Redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef]
- D’Suze, G.; Sandoval, M.; Sevcik, C. Characterizing Tityus discrepans scorpion venom from a fractal perspective: Venom complexity, effects of captivity, sexual dimorphism, differences among species. Toxicon 2015, 108, 62–72. [Google Scholar] [CrossRef]
- Rode-Margono, J.E.; Nekaris, K.A.-I. Cabinet of curiosities: Venom systems and their ecological function in mammals, with a focus on primates. Toxins 2015, 7, 2639–2658. [Google Scholar] [CrossRef] [Green Version]
- Casewell, N.R.; Wuester, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.E. Snake Venom Poisoning; J. B. Lippincott Company: Philadelphia, PA, USA, 1980; p. 562. [Google Scholar]
- Sunagar, K.; Casewell, N.R.; Varma, S.; Kolla, R.; Antunes, A.; Moran, Y. Deadly Innovations: Unraveling the Molecular Evolution of Animal Venoms; Springer: Dordrecht, The Netherlands, 2014; pp. 1–23. [Google Scholar] [CrossRef]
- Sunagar, K.; Moran, Y. The rise and fall of an evolutionary innovation: Contrasting strategies of venom evolution in ancient and young animals. PLoS Genet. 2015, 11, e1005596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The diversity of venom: The importance of behavior and venom sysmorphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [Green Version]
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [Green Version]
- Phuong, M.A.; Mahardika, G.N.; Alfaro, M.E. Dietary breadth is positively correlated with venom complexity in cone snails. BMC Genom. 2016, 17, 401. [Google Scholar] [CrossRef] [Green Version]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wuster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef] [Green Version]
- Daltry, J.C.; Wuster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Mebs, D. Snake venom composition and evolution of Viperidae. Kaupia 1999, 8, 145–148. [Google Scholar]
- Jansa, S.A.; Voss, R.S. Adaptive evolution of the venom-targeted vWF protein in opossums that eat pitvipers. PLoS ONE 2011, 6, e20997. [Google Scholar] [CrossRef] [PubMed]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Reumont, B.M.; Campbell, L.I.; Jenner, R.A. Quo Vadis Venomics? A Roadmap to Neglected Venomous Invertebrates. Toxins 2014, 6, 3488–3551. [Google Scholar] [CrossRef]
- Smith, W.L.; Stern, J.H.; Girard, M.G.; Davis, M.P. Evolution of venomous cartilaginous and ray-finned fishes. Integr. Comp. Biol. 2016, 56, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.A.; Mayhew, M.L.; Jin, J.Y.; Herzig, V.; Undheim, E.A.B.; Sombke, A.; Fry, B.G.; Meritt, D.J.; King, G.F. The assassin bug Pristhesancus plagipennis produces two distinct venoms in separate gland lumens. Nat. Commun. 2018, 9, 755. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, C. Venomous: How Earth’s Deadliest Creatures Mastered Biochemistry; Scientific American: New York, NY, USA, 2016. [Google Scholar]
- Rowe, A.H.; Rowe, M.P. Risk assessment by grasshopper mice (Onychomys spp.) feeding on neurotoxic prey (Centruroides spp.). Anim. Behav. 2006, 71, 725–734. [Google Scholar] [CrossRef]
- Schmidt, J.O. Hymenopteran venoms: Striving toward the ultimate defense against vertebrates. In Insect Defenses: Adaptive Mechanisms and Strategies of Prey and Predators; Evans, D.L., Ed.; State University of New York Press: Albany, NY, USA, 1990; pp. 383–417. [Google Scholar]
- Schmidt, J.O. Pain and lethality induced by insect stings: An exploratory and correlational study. Toxins 2019, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Jami, S.; Erickson, A.; Brierley, S.; Vetter, I. Pain-causing venom peptides: Insights into sensory neuron pharmacology. Toxins 2018, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Carcamo, E.; Olamendi-Portugal, T.; Restano-Cassulini, R.; Rowe, A.; Jocelyn Uribe-Romero, S.; Becerril, B.; Domingos Possania, L. Intraspecific Variation of Centruroides Sculpturatus Scorpion Venom from Two Regions of Arizona. Arch. Biochem. Biophys. 2018, 638, 52–57. [Google Scholar] [CrossRef]
- Santibanez-Lopez, C.E.; Francke, O.F.; Ureta, C.; Possani, L.D. Scorpions from Mexico: From species diversity to venom complexity. Toxins 2016, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.O. Venom and the good life in tarantula hawks (Hymenoptera: Pompilidae): How to eat, not be eaten, and live long. J. Kans. Entomol. Soc. 2004, 77, 402–413. [Google Scholar] [CrossRef]
- Waiddyanatha, S.; Silva, A.; Siribaddana, S.; Isbister, G.K. Long-term effects of snake envenoming. Toxins 2019, 11, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.S.V.; Silva, C.G.L.; Neto, B.S.; Grangeiro, C.R.P.; Lopes, V.H.G.; Teixeira, A.G.; Bezerra, D.A.; Luna, J.; Cordeiro, J.B.; Goncalves, J.; et al. Clinical and Epidemiological Aspects of Scorpionism in the World: A Systematic Review. Wilderness Environ. Med. 2016, 27, 504–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbuckle, K.; Rodriguez de la Vega, R.C.; Casewell, N.R. Coevolution takes the sting out of it: Evolutionary biology and mechanisms of toxin resistance in animals. Toxicon 2017, 140, 118–131. [Google Scholar] [CrossRef]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. R. Soc. B: Boil. Sci. 2015, 283, 20152841. [Google Scholar] [CrossRef]
- Holding, M.L.; Drabeck, D.H.; Jansa, S.A.; Gibbs, H.L. Venom resistance as a model for understanding the molecular basis of complex coevolutionary adaptations. Integr. Comp. Biol. 2016, 56, 1032–1043. [Google Scholar] [CrossRef]
- Poran, N.S.; Coss, R.G.; Benjamini, E. Resistance of California ground squirrels (Spermophilus beecheyi) to the venom of the northern Pacific rattlesnake (Crotalus viridis oreganus): A study of adaptive variation. Toxicon 1987, 25, 767–777. [Google Scholar] [CrossRef]
- Schmidt, J.O. Evolutionary responses of solitary and social Hymenoptera to predation by primates and overwhelmingly powerful vertebrate predators. J. Hum. Evol. 2014, 71, 12–19. [Google Scholar] [CrossRef]
- Schmidt, J.O. The Sting of the Wild; John Hopkins University Press: Baltimore, MD, USA, 2016; p. 257. [Google Scholar]
- Van der Meijden, A.; Koch, B.; van der Valk, T.; Vargas-Munoz, L.J.; Estrada-Gomez, S. Target-specificity in scorpions; comparing lethality of scorpion venoms across arthropods and vertebrates. Toxins 2017, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Summers, K.; Speed, M.P.; Blount, J.D.; Stuckert, A.M.M. Are aposematic signals honest? A review. J. Evol. Biol. 2015, 28, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J. Adaptive significance of venom glands in the tadpole madtom Noturus gyrinus (Siluriformes: Ictaluridae). J. Exp. Biol. 2012, 215, 1816–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brower, J.V.; Brower, L.P. Experimental studies of mimicry. 6. The reaction of toads (Bufo terrestris) to honeybees (Apis mellifera) and their dronefly mimics (Eristalis vinetorum). Am. Nat. 1962, 96, 297–307. [Google Scholar] [CrossRef]
- Brower, J.V.Z.; Brower, L.P. Experimental studies of mimicry. 8. Further investigations of honeybees (Apis mellifera) and their dronefly mimics (Eristalis spp.). Am. Nat. 1965, 99, 173–187. [Google Scholar] [CrossRef]
- Gall, B.G.; Spivey, K.L.; Chapman, T.L.; Delph, R.J.; Brodie, E.D.; Wilson, J.S. The indestructible insect: Velvet ants from across the United States avoid predation by representatives from all major tetrapod clades. Ecol. Evol. 2018, 8, 5852–5862. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.O.; Blum, M.S. Adaptations and responses of Dasymutilla occidentalis (Hymenoptera: Mutillidae) to predators. Entomol. Exp. Et Appl. 1977, 21, 99–111. [Google Scholar] [CrossRef]
- Vitt, L.J.; Cooper, W.E. Feeding responses of skinks (Eumeces laticeps) to velvet ants (Dasymutilla occidentalis). J. Herpetol. 1988, 22, 485–488. [Google Scholar] [CrossRef]
- Boyer, L.V.; Theodorou, A.A.; Berg, R.A.; Mallie, J.; Chávez-Méndez, A.; García-Ubbelohde, W.; Hardiman, S.; Alagón, A. Antivenom for critically ill children with neurotoxicity from scorpion stings. N. Engl. J. Med. 2009, 360, 2090–2098. [Google Scholar] [CrossRef] [Green Version]
- Gibly, R.; Williams, M.; Walter, F.G.; McNally, J.; Conroy, C.; Berg, R.A. Continuous intravenous midazolam infusion for Centruroides exilicauda scorpion envenomation. Ann. Emerg. Med. 1999, 34, 620–625. [Google Scholar] [CrossRef]
- Likes, K.; Banner, W.; Chavez, M. Centruroides exilicauda envenomation in Arizona. West. J. Med. 1984, 141, 634–637. [Google Scholar]
- LoVecchio, F.; McBride, C. Scorpion envenomations in young children in central Arizona. J. Toxicol. Clin. Toxicol. 2003, 41, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Valdez-Cruz, N.A.; Merino, E.; Zurita, M.; Possani, L.D. Genes and peptides from the scorpion Centruroides sculpturatus Ewing, that recognize Na+-channels. Toxicon 2001, 39, 1893–1898. [Google Scholar] [CrossRef]
- Rodríguez de la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure–function relationships and evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez de la Vega, R.C.R.; Possani, L.D. Current views on scorpion toxins specific for K+-channels. Toxicon 2004, 43, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Meves, H.; Watt, D.D. Neurtoxins in venom from the North American scorpion, Centruroides sculpturatus Ewing. In Natural Toxins: Toxicology, Chemistry, and Safety; Keeler, R.F., Mandava, N.B., Tu, A.T., Eds.; Alaken Inc.: Fort Collins, CO, USA, 1992; pp. 236–263. [Google Scholar]
- Rowe, A.H.; Xiao, Y.; Rowe, M.P.; Cummins, T.R.; Zakon, H.H. Voltage-gated sodium channel in grasshopper mice defends against bark scorpion toxin. Science 2013, 342, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldin, A.L.; Barchi, R.L.; Caldwell, J.H.; Hofmann, F.; Howe, J.R.; Hunter, J.C.; Kallen, R.G.; Mandel, G.; Meisler, M.H.; Netter, Y.B.; et al. Nomenclature of voltage-gated sodium channels. Neuron 2000, 28, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Zakon, H.H. Adaptive evolution of voltage-gated sodium channels: The first 800 million years. Proc. Natl. Acad. Sci. USA 2012, 109, 10619–10625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehesa-Dávila, M.; Possani, L.D. Scorpionism and serotherapy in Mexico. Toxicon 1994, 32, 1015–1018. [Google Scholar] [CrossRef]
- Parigi, A.; Thomas, L.; Fehrman, J.; Rowe, M.P.; Lubischer, J.L.; Rowe, A.H. Molecular mechanisms underlying scorpion mice (Onychomys torridus) muscle sodium channel (Nav1.4) resistance to bark scorpion (Centruroides sculpturatus) toxins drives structural variation in domains I, III and C-terminal; (Manuscript in preparation).
- Rowe, A.H.; Rowe, M.P. Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon 2008, 52, 597–605. [Google Scholar] [CrossRef]
- Stahnke, H.L. Arizona’s lethal scorpion Ariz. Med. 1972, 29, 490–493. [Google Scholar]
- Rowe, A.H.; Rowe, M.P. Assessment of toxic burden by scorpion mice (Onychomys torridus); (Manuscript in preparation).
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain 2007, 131, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef] [Green Version]
- Rowe, A.H.; Xiao, Y.; Scales, J.; Linse, K.D.; Rowe, M.P.; Cummins, T.R.; Zakon, H.H. Isolation and characterization of CvIV4: A pain inducing α-scorpion toxin. PLoS ONE 2011, 6, e23520. [Google Scholar] [CrossRef] [PubMed]
- Fet, V.; Lowe, G. Family Buthidae. In Catalog Of The Scorpions Of The World (1758–1998); Fet, V., Sissom, W.D., Lowe, G., Braundwalder, M.E., Eds.; The New York Entomological Society: New York, NY, USA, 2000; pp. 54–286. [Google Scholar]
- Holling, C.S. The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Vermeij, G.J. Unsuccessful Predation and Evolution. Am. Nat. 1982, 120, 701–720. [Google Scholar] [CrossRef]
- Langley, W.M. Relative importance of the distance senses in grasshopper mouse predatory behavior. Anim. Behav. 1983, 31, 199–205. [Google Scholar] [CrossRef]
- Barnett, C.A.; Skelhorn, J.; Bateson, M.; Rowe, C. Educated predators make strategic decisions to eat defended prey according to their toxin content. Behav. Ecol. 2012, 23, 418–424. [Google Scholar] [CrossRef]
- Mukherjee, S.; Heithaus, M.R. Dangerous prey and daring predators: A review. Biol. Rev. 2013, 88, 550–563. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef] [Green Version]
- Skinner, B.F.; Campbell, S.L. An automatic shocking-grid apparatus for continuous use. J. Comp. Physiol. Psychol. 1947, 40, 305–307. [Google Scholar] [CrossRef]
- Bali, A.; Jaggi, A.S. Electric foot shock stress: A useful tool in neuropsychiatric studies. Rev. Neurosci. 2015, 26, 655–677. [Google Scholar] [CrossRef]
- Boe, E.E. Extinction as a function of intensity of punishment, amount of training, and reinforcement of a competing response. Can. J. Psychol. 1964, 18, 328–342. [Google Scholar] [CrossRef]
- Boe, E.E.; Church, R.M. Permanent effects of punishment during extinction. J. Comp. Physiol. Psychol. 1967, 63, 486–492. [Google Scholar] [CrossRef]
- Skelhorn, J.; Halpin, C.G.; Rowe, C. Learning about aposematic prey. Behav. Ecol. 2016, 27, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Launchbaugh, K.L.; Provenza, F.D. Can plants practice mimicry to avoid grazing by mammalian herbivores? Oikos 1993, 66, 501–504. [Google Scholar] [CrossRef]
- Provenza, F.D.; Kimball, B.A.; Villalba, J.J. Roles of odor, taste, and toxicity in the food preferences of lambs: Implications for mimicry in plants. Oikos 2000, 88, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.A.; Bowen, A.J.; Roman, C.W.; Palmiter, R.D. Encoding of danger by parabrachial CGRP neurons. Nature 2018, 555, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Lemon, C.H. Mouse Parabrachial Neurons Signal a Relationship between Bitter Taste and Nociceptive Stimuli. J. Neurosci. 2019, 39, 1631–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhadeff, A.L.; Su, Z.W.; Hernandez, E.; Klima, M.L.; Phillips, S.Z.; Holland, R.A.; Guo, C.Y.; Hantman, A.W.; De Jonghe, B.C.; Betley, J.N. A neural circuit for the suppression of pain by a competing need state. Cell 2018, 173, 140–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledue, E.E.; Mann, K.; Koch, E.; Chu, B.; Dakin, R.; Gordon, M.D. Starvation-induced depotentiation of bitter taste in Drosophila. Curr. Biol. 2016, 26, 2854–2861. [Google Scholar] [CrossRef] [Green Version]
- Cyr, M.A. Predatory Behavior of the Grasshopper Mouse (Onychomys). Doctoral Dissertation, University of California, Los Angeles, CA, USA, 1972. [Google Scholar]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Langley, W.M. Development of predatory behavior in the southern grasshopper mouse (Onychomys torridus). Behaviour 1986, 99, 275–295. [Google Scholar] [CrossRef]
- Secor, S.M.; Wooten, J.A.; Cox, C.L. Effects of meal size, meal type, and body temperature on the specific dynamic action of anurans. J. Comp. Physiol. B 2007, 177, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1984; p. 718. [Google Scholar]
- Keppel, G. Design and Analysis: A Researcher’s Handbook, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1982. [Google Scholar]
- Whitlock, M.C. Combining probability from independent tests: The weighted Z-method is superior to Fisher’s approach. J. Evol. Biol. 2005, 18, 1368–1373. [Google Scholar] [CrossRef]


| Type of Trial | Stage of Predatory Sequence 1 | Dependent Variable 2 | Definition |
|---|---|---|---|
| Single-cup training trials | Handling Time | Elapsed time between a mouse’s first attack (see below) and the moment the scorpion was no longer capable of fighting back or escaping | |
| Double-cup preference trials | Search | Approach | Mouse moves to within ½ body length of cup containing scorpion |
| Search | Inspect | Mouse sticks nose over the top of the cup, breaking an imaginary vertical plane at the edge of the cup | |
| Pursuit | Tip | Mouse tips the cup on its side | |
| Attack | Attack | Mouse attempts to capture scorpion by lunging with forepaws outstretched and mouth open | |
| Consume | Consume | Mouse begins eating the scorpion |
| Experiment #1 | Experiment #2 | Experiment #3 | |
|---|---|---|---|
| Objective | mimic natural encounters in the field | control for scorpion mass but not sting painfulness | control for both scorpion mass and sting painfulness |
| AZ bark-scorpion mass | 0.555 ± 0.018 g (n = 72) | 0.784 ± 0.017 g (n = 98) | 0.48 ± 0.010 g (n = 145) |
| Stripe-tailed scorpion mass | 0.838 ± 0.031 g (n = 72) | 0.784 ± 0.017 g (n = 98) | 0.47 ± 0.010 g (n = 143) |
| Mass differences between scorpion species | t = 7.82, DF = 142 p < 0.0001 r2 = 0.301 | t = 0.026, DF = 194 p = 0.979 | t = 0.641, DF = 284 p = 0.522 |
| AZ bark scorpion stinger |  |  |  |
| Stripe-tailed scorpion stinger |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niermann, C.N.; Tate, T.G.; Suto, A.L.; Barajas, R.; White, H.A.; Guswiler, O.D.; Secor, S.M.; Rowe, A.H.; Rowe, M.P. Defensive Venoms: Is Pain Sufficient for Predator Deterrence? Toxins 2020, 12, 260. https://doi.org/10.3390/toxins12040260
Niermann CN, Tate TG, Suto AL, Barajas R, White HA, Guswiler OD, Secor SM, Rowe AH, Rowe MP. Defensive Venoms: Is Pain Sufficient for Predator Deterrence? Toxins. 2020; 12(4):260. https://doi.org/10.3390/toxins12040260
Chicago/Turabian StyleNiermann, Crystal N., Travis G. Tate, Amber L. Suto, Rolando Barajas, Hope A. White, Olivia D. Guswiler, Stephen M. Secor, Ashlee H. Rowe, and Matthew P. Rowe. 2020. "Defensive Venoms: Is Pain Sufficient for Predator Deterrence?" Toxins 12, no. 4: 260. https://doi.org/10.3390/toxins12040260
APA StyleNiermann, C. N., Tate, T. G., Suto, A. L., Barajas, R., White, H. A., Guswiler, O. D., Secor, S. M., Rowe, A. H., & Rowe, M. P. (2020). Defensive Venoms: Is Pain Sufficient for Predator Deterrence? Toxins, 12(4), 260. https://doi.org/10.3390/toxins12040260