Iron Deposits in Periaqueductal Gray Matter Are Associated with Poor Response to OnabotulinumtoxinA in Chronic Migraine
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Sample
2.1.1. Demographics
- Non-responders were significantly older than responders (mean age difference = 12.2; 95% confidence interval (CI): 5.4–18.9, p = 0.001).
- No statistically significant differences were observed regarding gender, body mass index, smoking habit, medication, or time of evolution of chronic migraine.
2.1.2. Characteristics of Migraine
- Intensity (mean visual analogic scale (VAS) difference = 0.4; 95% CI: 0.5–1.5, p = 0.242), duration (mean difference in hours = 3.7; 95% CI: 18.2–25.6, p = 0.554), and frequency (mean difference in days/month = 4.7; 95% CI: 0.08–9.3, p = 0.127) of migraine attacks were similar in both responders and non-responders.
- No differences were found for the presence of aura, allodynia, or tension-type headache.
- Within the group of responders, 36 (76.6%) were categorized as moderate responders while 11 (23.4%) showed an excellent response to OnabotA.
2.2. Predictors of Response
2.2.1. Molecular Biomarkers
- Thirty-eight out of 47 (80.9%) chronic migraineurs showing good response to OnabotA presented significantly higher serum levels of CGRP (≥50 ng/mL) compared to 4 out of 15 (26.7%) with a poor outcome.
- Similarly, 87.2% (41/47) of responders had elevated serum levels of PTX3 (≥1000 pg/mL) in comparison to 20.0% (3/15) of non-responders.
2.2.2. Imaging Biomarkers
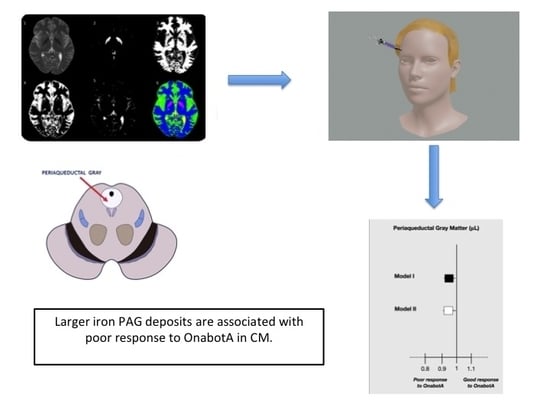
- Statistically significant differences between responders and non-responders were found for iron deposition in the GP and PAG (mean difference = 805.0; 95% CI: 37.9–1572.1 μL, p = 0.040 and mean difference = 69.8; 95% CI: 31.0–108.6 μL, p = 0.008; respectively). Adjustment for age in the multivariate model changed statistical significance for GP (mean difference = 472.4; 95% CI: 341.5–1286.4 μL, p = 0.250) but not for PAG (mean difference = 65.7; 95% CI: 22.8–108.6 μL, p = 0.003).
- No discrepancies were observed for the prevalence, number, and location of WML between responders and non-responders (Table 2).
- Iron deposition in the PAG was associated with higher odds of poor response to OnabotA (Figure 1). A 10% increase in iron ground volumes in the PAG was associated with an odds ratio for poor response to treatment of 0.973 (95% CI: 0.955–0.991, p = 0.040) independently of age and GP (Figure 1, Model I; Table 3).
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Protocol
5.2. Laboratory Tests
5.3. Neuroimaging Variables
5.4. Standard Protocol Approvals, Registrations, and Patient Consents
5.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- May, A.; Goadsby, P.J. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J. Cereb. Blood Flow Metab. 1999, 19, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Levy, D.; Kainz, V.; Noseda, R.; Jakubowski, M.; Burstein, R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011, 69, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Durham, P.; Papapetropoulos, S. Biomarkers associated with migraine and their potential role in migraine management. Headache 2013, 53, 1262–1277. [Google Scholar] [CrossRef]
- Kurth, T.; Gaziano, J.M.; Cook, N.R.; Logroscino, G.; Diener, H.C.; Buring, J.E. Migraine and risk of cardiovascular disease in women. JAMA 2006, 296, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Gursoy-Ozdemir, Y.; Qiu, J.; Matsuoka, N.; Bolay, H.; Bermpohl, D.; Jin, H.; Wang, X.; Rosenberg, G.A.; Lo, E.H.; Moskowitz, M.A. Cortical spreading depression activates and upregulates MMP-9. J. Clin. Investig. 2004, 113, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd ed. (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurora, S.K.; Winner, P.; Freeman, M.C.; Spierings, E.L.; Heiring, J.O.; DeGryse, R.E.; van Denburgh, A.M.; Nolan, M.E.; Turkel, C.C. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache 2011, 9, 1358–1373. [Google Scholar] [CrossRef]
- Burstein, R.; Zhang, X.; Levy, D.; Aoki, K.R.; Brin, M.F. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia 2014, 34, 853–869. [Google Scholar] [CrossRef]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Manack Adams, A.; Brin, M.F. Mechanism of action of OnabotulinumtoxinA in chronic migraine: A narrative review. Headache 2020. [Google Scholar] [CrossRef]
- Aurora, S.K.; Dodick, D.W.; Turkel, C.; DeGryse, R.; Silberstein, S.; Lipton, R.; Diener, H.; Brin, M. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.; Dodick, D.W.; Aurora, S.K.; Turkel, C.; DeGryse, R.; Lipton, R.; Silberstein, S.; Brin, M. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010, 50, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Oterino, A.; Ramón, C.; Pascual, J. Experience with onabotulinumtoxinA (Botox) in chronic refractory migraine: Focus on severe attacks. J. Headache Pain 2011, 12, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Sandrini, G.; Perrotta, A.; Tassorelli, C.; Torelli, P.; Brighina, G.; Sances, G.; Nappi, G. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: A multicenter, double-blind, randomized, placebo-controlled, parallel group study. J. Headache Pain 2011, 12, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.; Zafar, H.W.; Quarshie, V.; Ahmed, F. Prospective analysis of the use of OnabotulinumtoxinA (BOTOX) in the treatment of chronic migraine; real-life data in 254 patients from Hull, UK. J. Headache Pain 2014, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Cernuda-Morollón, E.; Ramón, C.; Larrosa, D.; Alvarez, R.; Riesco, N.; Pascual, J. Long-term experience with OnabotulinumtoxinA in the treatment of chronic migraine: What happens after one year? Cephalalgia 2015, 35, 864–868. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Aurora, S.K.; Diener, H.C.; DeGryse, R.E.; Lipton, R.B.; Turkel, C.C. Per cent of patients with chronic migraine who responded per OnabotulinumtoxinA treatment cycle:PREEMPT. J. Neurol. Neurosurg. Psychiatry 2015, 86, 996–1001. [Google Scholar] [CrossRef]
- Pedraza, M.I.; de la Cruz, C.; Ruiz, M.; López-Mesonero, L.; Martínez, E.; de Lera, M.; Guerrero, A.L. OnabotulinumtoxinA treatment for chronic migraine: Experience in 52 patients treated with the PREEMPT paradigm. Springerplus 2015, 4, 176. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Manzoni, G.C.; Taga, A.; Genovese, A.; Veronesi, L.; Pasquarella, C.; Sansebastiano, G.E.; Torelli, P. The use of onabotulinum toxin A (Botox®) in the treatment of chronic migraine at the Parma Headache Centre: A prospective observational study. Neurol. Sci. 2016, 37, 1127–1131. [Google Scholar] [CrossRef]
- Aicua-Rapun, I.; Martinez-Velasco, E.; Rojo, A.; Hernando, A.; Ruiz, M.; Carreres, A.; Porqueres, E.; Herrero, S.; Iglesias, F.; Guerrero, A.L. Real-life data in 115 chronic migraine patients treated with Onabotulinumtoxin A during more than one year. J. Headache Pain 2016, 17, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, C.; Pozo-Rosich, P.; Torres-Ferrus, M.; Hernandez-Beltrán, N.; Jurado-Cobo, C.; Gonzalez-Oria, C.; Santos, S.; Monzón, M.J.; Latorre, G.; Alvaro, L.C.; et al. OnabotulinumtoxinA in chronic migraine: Predictors of response. A prospective multicentre descriptive study. Eur. J. Neurol. 2018, 25, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.; Varon, S.; Grosberg, B.; McAllister, P.; Freitag, F.; Aurora, S.; Dodick, D.W.; Silberstein, S.; Diener, H.; DeGryse, R.; et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology 2011, 77, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Kollewe, K.; Escher, C.M.; Wulff, D.U.; Fathi, D.; Paracka, L.; Mohammadi, B.; Karst, M.; Dressler, D. Long-term treatment of chronic migraine with Onabotulinum- toxinA: Efficacy, quality of life and tolerability in a real- life setting. J. Neural. Transm. 2016, 123, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mathew, N.T.; Kailasam, J.; Meadors, L. Predictors of response to botulinum toxin type A (BoNTA) in chronic daily headache. Headache 2008, 48, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Eross, E.J.; Gladstone, J.P.; Lewis, S.; Rogers, R.; Dodick, D.W. Duration of migraine is a predictor for response to botulinum toxin type A. Headache 2005, 45, 308–314. [Google Scholar] [CrossRef]
- Jakubowski, M.; McAllister, P.J.; Bajwa, Z.H.; Ward, T.N.; Smith, P.; Burstein, R. Exploding vs. imploding headache in migraine prophylaxis with Botulinum Toxin A. Pain 2006, 125, 286–295. [Google Scholar] [CrossRef]
- Di Cola, S.; Caratozzolo, S.; Rao, R.; Padovani, A. Response predictors in chronic migraine: Medication overuse and depressive symptoms negatively impact OnabotulinumtoxinA treatment. Front. Neurol. 2019, 10, 678. [Google Scholar] [CrossRef]
- Pagola, I.; Esteve-Belloch, P.; Palma, J.; Luquin, M.; Riverol, M.; Martinez-Vila, E.; Irimia, P. Predictive factors of the response to treatment with onabotulinumtoxinA in refractory migraine. Rev. Neurol. 2014, 58, 241–246. [Google Scholar]
- Lin, K.H.; Chen, S.P.; Fuh, J.L.; Wang, Y.F.; Wang, S.J. Efficacy, safety and predictors of response to botulinum toxin type A in refractory chronic headache: A retrospective study. J. Chin. Med. Assoc. 2014, 77, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Lee, C.; Choi, H.; Chung, C.S. Factors associated with favorable outcome in botulinum toxin A treatment for chronic migraine: A clinic-based prospective study. J. Neurol. Sci. 2016, 363, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Ferroni, P. Onabotulinum toxinA in the treatment of chronic migraine: Patient selection and special considerations. J. Pain Res. 2017, 10, 2319–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, C.S.; Becerra, L.; Smith, J.H.; DeLange, J.M.; Smith, R.M.; Black, D.F.; Welker, K.M.; Burstein, R.; Cutter, F.M.; Borsook, D. Brain changes in responders vs. non-responders in chronic migraine: Markers of disease reversal. Front. Hum. Neurosci. 2016, 10, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumb, A.; Seifert, B.; Wetzel, S.; Agosti, R. Patients profiling for Botox (onabotulinum toxin A) treatment for migraine: A look at white matter lesions in the MRI as a potential marker. Springer Plus 2013, 2, 377. [Google Scholar] [CrossRef] [Green Version]
- Cady, R.; Turner, I.; Dexter, K.; Beach, M.E.; Cady, R.; Durham, P. An exploratory study of salivary calcitonin gene-related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache 2014, 54, 269–277. [Google Scholar] [CrossRef]
- Cernuda-Morollon, E.; Martinez-Camblor, P.; Ramon, C.; Larrosa, D.; Serrano-Pertierra, E.; Pascual, J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 2014, 54, 987–995. [Google Scholar] [CrossRef]
- Dominguez, C.; Vieites-Pardo, A.; Perez-Mato, M.; Sobrino, T.; Rodriguez-Osorio, X.; Lopez, A.; Campos, F.; Martinez, F.; Castillo, J.; Leira, R. CGRP and PTX3 as predictors of efficacy of Onambotulinumtoxin type A in chronic migraine: An observational study. Headache 2018, 58, 78–87. [Google Scholar] [CrossRef]
- Domínguez-Vivero, C.; Leira, Y.; López-Ferreiro, A.; Saavedra, M.; Rodríguez-Osorio, X.; Sobrino, T.; Campos, F.; Castillo, J.; Leira, R. Pentraxin 3 (PTX3): A molecular marker of endothelial dysfunction in chronic migraine. J. Clin. Med. 2020, 9, 849. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, C.; Lopez, A.; Ramos-Cabrer, P.; Vieites-Prado, A.; Perez-Mato, M.; Villalba, C.; Sobrino, T.; Rodriguez-Osorio, X.; Campos, F.; Castillo, J.; et al. Iron deposition in periaqueductal gray matter as a potential biomarker for chronic migraine. Neurology 2019, 92, 1–10. [Google Scholar] [CrossRef]
- Rothrock, A.; Parada, J.F.; Drinkard, V.A.; Zweifler, R.M.; Key, K.F. Predictors of a negative response to topiramate therapy in patients with chronic migraine. Headache 2005, 45, 932–935. [Google Scholar] [CrossRef]
- Alpuente, A.; Gallardo, V.J.; Torres-Ferrús, M.; Lasaosa-Santos, S.; Guerrero, A.L.; Lainez, J.M.; Viguera, J.; Gago-Veiga, A.; Irimia, P.; Sanchez del Rio, M.; et al. Evaluation of the concomitant use of oral preventive treatments and OnabotulinumtoxinA in chronic migraine: The PREVENBOX study. Eur. J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.D.; Scott, D.J.; Zubieta, J.K. Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. USA 2007, 104, 11056–11061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, K.M.; Nagesh, V.; Aurora, S.K.; Gelman, N. Periaqueductal gray matter dysfunction in migraine: Cause or the burden of illness? Headache 2001, 41, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, M.; Wiech, K.; Dunckley, P.; Tracey, I. Anticipatory brainstem activity predicts neural processing of pain in humans. Eur. J. Pain 2006, 10, S83b. [Google Scholar] [CrossRef]
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Raskin, N.H.; Yoshio, H.; Sharon, L. Headache may arise from perturbation of brain. Headache 1987, 27, 416–420. [Google Scholar] [CrossRef]
- Tepper, S.J.; Lowe, M.J.; Beall, E.; Phillips, M.D.; Liu, K.; Stillman, M.J.; Horvat, M.; Jones, S.E. Iron deposition in pain-regulatory nuclei in episodic migraine and chronic daily headache by MRI. Headache 2012, 52, 236–243. [Google Scholar] [CrossRef]
- Palm-Meinders, I.H.; Koppen, H.; Terwindt, G.M.; Launer, L.J.; van Buchem, M.A.; Ferrari, M.D.; Kruit, M. Iron in deep brain nuclei in migraine? CAMERA follow-up MRI findings. Cephalalgia 2017, 37, 795–800. [Google Scholar] [CrossRef]
- Kruit, M.C.; Launer, L.J.; Overbosch, J.; van Buchem, M.A.; Ferrari, M.D. Iron accumulation in deep brain nuclei in migraine: A population-based magnetic resonance imaging study. Cephalalgia 2009, 29, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Haider, L. Inflammation, iron, energy failure, and oxidative stress in the pathogenesis of multiple sclerosis. Oxid Med. Cell. Longev. 2015, 2015, 725370. [Google Scholar] [CrossRef]
- Swaiman, K.F.; Machen, V.L. Iron uptake by glial cells. Neurochem. Res. 1985, 10, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Burdo, J.R.; Simpson, I.A.; Menzies, S.; Beard, J.; Connor, J.R. Regulation of the profile of iron-management proteins in brain microvasculature. J. Cereb. Blood Flow Metab. 2004, 24, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar] [CrossRef]
- Linker, R.A.; Kroner, A.; Horn, T.; Mäurer, M.; Bendszus, M. Iron particle-enhanced visualization of inflammatory central nervous system lesions by high resolution: Preliminary data in an animal model. AJNR Am. J. Neuroradiol. 2006, 27, 1225–1229. [Google Scholar] [PubMed]
- Williams, S.; Rohr, A.M.; Wang, W.T.; Choi, I.Y.; Lee, P.; Berman, N.E.J.; Lynch, S.G.; LeVine, S.M. Iron deposition is independent of cellular inflammation in a cerebral model of multiple sclerosis. BMC Neurosci. 2011, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, C.M.; Candy, J.M.; Omar, S.; Bloxham, C.A.; Edwardson, J.A. Transferrin receptors in the parkinsonian midbrain. Neuropathol. Appl. Neurobiol. 1994, 20, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, S.A.; Connor, J.R. Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J. Comp. Neurol. 1993, 338, 97–113. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef]
- Won, S.M.; Lee, J.H.; Park, U.J.; Gwag, J.; Gwag, B.J.; Lee, Y.B. Iron mediates endothelial cell damage and blood-brain barrier opening in the hippocampus after transient forebrain ischemia in rats. Exp. Mol. Med. 2011, 43, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Bartzokis, G.; Tishler, T.A.; Lu, P.H.; Villablanca, P.; Altshuler, L.L.; Carter, M.; Huang, D.; Edwards, N.; Mintz, J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging 2007, 28, 414–423. [Google Scholar] [CrossRef]
- Bilgic, B.; Pfefferbaum, A.; Rohlfing, T.; Sullivan, E.V.; Adalsteinsson, E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage 2012, 59, 2625–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherubini, A.; Peran, P.; Caltagirone, C.; Sabatini, U.; Spalletta, G. Aging of subcortical nuclei: Microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage 2009, 48, 29. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Adalsteinsson, E.; Rohlfing, T.; Sullivan, E.V. MRI estimates of brain iron concentration in normal aging: Comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage 2009, 47, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, N.; Wu, J.; Zhang, Q.; Liu, T.; Shen, J.; Bao, R.; Ni, M.; Wang, Y.; Spincemaille, P. Age and sex related differences in subcortical brain iron concentrations among healthy adults. Neuroimage 2015, 122, 385–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasband, W.S. Image J. USA National Institutes of Health: Bethesda, Maryland, USA, 1997–2014. Available online: http://imagej.nih.gov/ij/ (accessed on 15 March 2016).
- Jurgens, C.K.; Jasinschi, R.; Ekin, A.; Witjes-Ane, M.N.W.; van der Grond, J.; Middelkoop, H.; Raymund, A.C.R. MRI T2 Hypointensities in basal ganglia of premanifest Huntington’s disease. PLoS Curr. 2010, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Variables | Responders (n = 47) | Non Responders (n = 15) | p-Value |
|---|---|---|---|
| Age (years) Gender, Females, n (%) | 39.4 ± 12.0 46 (97.9) | 51.6 ± 9.1 14 (93.3) | 0.001 0.428 |
| Body mass index (kg/m2) | 24.8 [22.6, 27.4] | 25.5 [23.7, 28.9] | 0.425 |
| Allodynia, n (%) | 15 (31.9) | 8 (53.3) | 0.135 |
| Aura, n (%) | 22 (46.8) | 9 (60.0) | 0.554 |
| Tension-type headache, n (%) | 25 (53.2) | 10 (66.7) | 0.537 |
| Preventive treatment (≥2 drugs), n (%) | 24 (51.0) | 8 (53.4) | 0.117 |
| Symptomatic treatment (≥2 drugs), n (%) | 29 (61.7) | 8 (53.3) | 0.749 |
| Neuroimaging Variables | Responders (n = 47) | Non Responders (n = 15) | p-Value |
|---|---|---|---|
| Iron deposits (μL) | |||
| Red Nucleus (median [interquartile range]) | 39.6 [3.5, 99.0] | 83.7 [19.3, 128.3] | 0.244 |
| Substantia Nigra (median [interquartile range]) | 205.6 [105.0, 397.4] | 257.4 [158.8, 607.0] | 0.305 |
| Globus Pallidus (mean ± standard deviation) | 1690.4 ± 995.5 | 2495.5 ± 1852.3 | 0.040 |
| Periaqueductal Gray Matter (median [interquartile range]) | 352.0 [265.2, 365.7] | 455.5 [408.5, 473.5] | 0.008 |
| Presence of White Matter Lesions, n (%) | 24 (51.1) | 11 (73.3) | 0.130 |
| Number of White Matter Lesions | |||
| <3, n (%) | 3 (12.5) | 0. (0.0) | 0.230 |
| 3–6, n (%) | 13 (54.2) | 7 (63.6) | 0.620 |
| >6, n (%) | 8 (33.3) | 4 (36.4) | 0.840 |
| Location of White Matter Lesions | |||
| Subcortical, n (%) | 8 (33.3) | 4 (36.4) | 0.860 |
| Subcortical + periventricular, n (%) | 11 (45.8) | 7 (63.6) | 0.330 |
| Subcortical + other locations, n (%) | 5 (20.8) | 0 (0.0) | 0.100 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Model I | |||
| Age | 0.882 | 0.769–0.970 | 0.012 |
| Globus Pallidus (µL) | 0.999 | 0.997–1.002 | 0.613 |
| Periaqueductal gray (µL) | 0.973 | 0.955–0.991 | 0.040 |
| Model II | |||
| Age | 0.815 | 0.668–0.995 | 0.044 |
| CGRP ≥ 50 (ng/mL) | 1.026 | 1.001–1.050 | 0.034 |
| PTX3 ≥ 1000 (ng/mL) | 1.008 | 1.001–1.016 | 0.037 |
| Periaqueductal gray (µL) | 0.963 | 0.927–0.997 | 0.041 |
| Dependent variable: OnabotA response (good versus poor) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez Vivero, C.; Leira, Y.; Saavedra Piñeiro, M.; Rodríguez-Osorio, X.; Ramos-Cabrer, P.; Villalba Martín, C.; Sobrino, T.; Campos, F.; Castillo, J.; Leira, R. Iron Deposits in Periaqueductal Gray Matter Are Associated with Poor Response to OnabotulinumtoxinA in Chronic Migraine. Toxins 2020, 12, 479. https://doi.org/10.3390/toxins12080479
Domínguez Vivero C, Leira Y, Saavedra Piñeiro M, Rodríguez-Osorio X, Ramos-Cabrer P, Villalba Martín C, Sobrino T, Campos F, Castillo J, Leira R. Iron Deposits in Periaqueductal Gray Matter Are Associated with Poor Response to OnabotulinumtoxinA in Chronic Migraine. Toxins. 2020; 12(8):479. https://doi.org/10.3390/toxins12080479
Chicago/Turabian StyleDomínguez Vivero, Clara, Yago Leira, Marta Saavedra Piñeiro, Xiana Rodríguez-Osorio, Pedro Ramos-Cabrer, Carmen Villalba Martín, Tomás Sobrino, Francisco Campos, José Castillo, and Rogelio Leira. 2020. "Iron Deposits in Periaqueductal Gray Matter Are Associated with Poor Response to OnabotulinumtoxinA in Chronic Migraine" Toxins 12, no. 8: 479. https://doi.org/10.3390/toxins12080479
APA StyleDomínguez Vivero, C., Leira, Y., Saavedra Piñeiro, M., Rodríguez-Osorio, X., Ramos-Cabrer, P., Villalba Martín, C., Sobrino, T., Campos, F., Castillo, J., & Leira, R. (2020). Iron Deposits in Periaqueductal Gray Matter Are Associated with Poor Response to OnabotulinumtoxinA in Chronic Migraine. Toxins, 12(8), 479. https://doi.org/10.3390/toxins12080479