Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites
Abstract
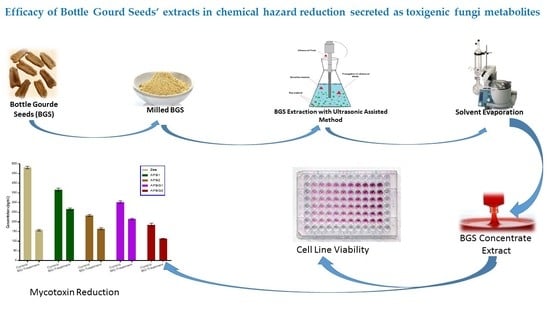
:1. Introduction
2. Results
2.1. Chemical Composition of Bottle Gourd Powder of the Seeds
2.2. Fatty Acid Composition, Tocols, Sterols, and Carotenoids Content of Extracted Oil
2.3. Antioxidant Activity of the BG-Polar Extract
2.4. Determination of Phenolic Fractions
2.5. Antifungal Activities for the Polar Extract of Bottle Gourd
2.6. Evaluation of Bottle Gourd Extract Healthy Impact
2.7. Reduction Impact of BG-Polar Extract for Mycotoxin Production
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals, Reagents, and Sample Collection
5.2. Preparation of Polar and Non-Polar Extracts
5.3. Determination of Total Phenolic and Flavonoid Contents (TPC)
5.4. Determination of Fatty Acid Profile
5.5. Determination of Tocopherol and Tocotrienol Contents
5.6. Determination of Sterols and Carotenoid Content
5.7. Antioxidant Activity for Polar Extract
5.7.1. Scavenging Activity of DPPH Radicals
- Abs 517 (control): The absorbance of the blank at 517 wavelengths.
- Abs 517 (sample): The absorbance of the determined sample at 517 wavelengths.
5.7.2. Determination of Reducing Power
5.7.3. Antioxidant Activities Using Scavenging of Hydroxyl Radicals
- AS: Is the absorbance of the sample
- A0: Is the absorbance of the control
- A: Is the absorbance without sample or Fenton reaction system.
5.8. Determination of Phenolic Fractions of Polar Extract
5.9. Estimation of Antifungal Activity of the Polar BG Extract
5.9.1. Agar Diffusion Assay
5.9.2. Fungal-Growth Inhibition
- Control: media just contained inoculated fungi
- Treated: media contains the BG extract with inoculated fungi.
5.9.3. Toxin Production Reduction in Liquid Media
5.10. Evaluation of Bottle Gourd Extract Healthy Impact
5.11. Aflatoxins and Zearalenone Determinations
5.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.B.; Attar, U.A.; Sakate, D.M.; Ghane, S.G. Efficient extraction of cucurbitacins from Diplocyclos palmatus (L.) C. Jeffrey: Optimization using response surface methodology, extraction methods and study of some important bioactivities. Sci. Rep. 2020, 10, 2109. [Google Scholar] [CrossRef] [PubMed]
- Ghane, S.G.; Attar, U.A.; Yadav, P.B.; Lekhak, M.M. Antioxidant, anti-diabetic, acetylcholinesterase inhibitory potential and estimation of alkaloids (lycorine and galanthamine) from Crinum species: An important source of anticancer and anti-Alzheimer drug. Ind. Crop. Prod. 2018, 125, 168–177. [Google Scholar] [CrossRef]
- Adewale, O.O.; Oduyemi, O.I.; Ayokunle, O. Oral administration of leaf extracts of Momordica charantia affect reproductive hormones of adult female Wistar rats. Asian Pac. J. Trop. Biomed. 2014, 4, S521–S524. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 2005, 77, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.A.; Ali, S.M.; Siddiqui, M.; Khan, A.U. Antimicrobial Activity of Five Herbal Extracts Against Multi Drug Resistant (MDR) Strains of Bacteria and Fungus of Clinical Origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef]
- Krawinkel, M.B.; Keding, G.B. Bitter Gourd (Momordica charantia): A Dietary Approach to Hyperglycemia. Nutr. Rev. 2006, 64, 331–337. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Hsu, C.; Chao, C.-Y.; Wein, Y.-S.; Kuo, Y.-H.; Huang, C.-J. Fractionation and identification of 9c, 11t, 13t-conjugated linolenic acid as an activator of PPARα in bitter gourd (Momordica charantia L.). J. Biomed. Sci. 2006, 13, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Kobori, M.; Nakayama, H.; Fukushima, K.; Ohnishi-Kameyama, M.; Ono, H.; Fukushima, T.; Akimoto, Y.; Masumoto, S.; Yukizaki, C.; Hoshi, Y.; et al. Bitter Gourd Suppresses Lipopolysaccharide-Induced Inflammatory Responses. J. Agric. Food Chem. 2008, 56, 4004–4011. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.S.; Grover, J.K.; Vikrant, V.; Biswas, N.R. Prevention of experimental diabetic cataract by Indian Ayurvedic plant extracts. Phytotheraby Res. 2002, 16, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Attar, U.A.; Ghane, S.G. In vitro antioxidant, antidiabetic, antiacetylcholine esterase, anticancer activities and RP-HPLC analysis of phenolics from the wild bottle gourd (Lagenaria siceraria (Molina) Standl.). S. Afr. J. Bot. 2019, 125, 360–370. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; Marcone, M.F. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process. Preserv. 2021, 45, e15112. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Shehata, M.G.; Badr, A.N.; Gromadzka, K.; Stępień, L. The effect of chemical composition of wild Opuntia ficus indica byproducts on its nutritional quality, antioxidant and antifungal efficacy. Egypt. J. Chem. 2019, 62, 47–61. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G. Prospective antimycotoxigenic action of wild Opuntia ficus-indica by-products. Czech J. Food Sci. 2020, 38, 308–314. [Google Scholar] [CrossRef]
- Sabry, B.A.; Farouk, A.; Badr, A.N. Bioactivity evaluation for volatiles and water extract of commercialized star anise. Heliyon 2021, 7, e07721. [Google Scholar] [CrossRef]
- Badr, A.N.; Shehata, M.G.; Abdel-Razek, A.G. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; El-Said, M.M.; El-Messery, T.M.; Abdel-Razek, A.G. Non-traditional oils encapsulation as novel food additive enhanced yogurt safety against aflatoxins. Pak. J. Biol. Sci. 2019, 22, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, A.; Tulika, P.; Singh, A. Study on the impact of selected contact and systemic fungicides on mycelial growth of Alternari alternata in vitro. Indian J. Sci. Res. 2017, 14, 314–319. [Google Scholar]
- Ahmad, I.; Irshad, M.; Rizvi, M.M.A. Nutritional and Medicinal Potential of Lagenaria siceraria. Int. J. Veg. Sci. 2011, 17, 157–170. [Google Scholar] [CrossRef]
- Ogunbusola, E.M. Nutritional and Antinutritional Composition of Calabash and Bottle Gourd Seed Flours (var Lagenaria siceraria). J. Culin. Sci. Technol. 2018, 16, 326–335. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic Acid Bacteria as a Cell Factory for the Delivery of Functional Biomolecules and Ingredients in Cereal-Based Beverages: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, A.M.; Badr, A.N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef]
- da Silva, E.O.; Bracarense, A.P.; Oswald, I.P. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Zhao, Y.-Y.; Xiong, B.; Cui, X.-S.; Kim, N.-H.; Xu, Y.-X.; Sun, S.-C. Mycotoxin-Containing Diet Causes Oxidative Stress in the Mouse. PLoS ONE 2013, 8, e60374. [Google Scholar] [CrossRef]
- Ou, S.-Y.; Jackson, G.M.; Jiao, X.; Chen, J.; Wu, J.-Z.; Huang, X.-S. Protection against Oxidative Stress in Diabetic Rats by Wheat Bran Feruloyl Oligosaccharides. J. Agric. Food Chem. 2007, 55, 3191–3195. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Functional oligosaccharides: Production, properties and applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of N-3 polyunsaturated fatty acid inhibition of mycotoxin deoxynivalenol-induced immune response. J. Interferon Cytokine Res. 1999, 29, 250–252. [Google Scholar]
- Calvo, A.; Hinze, L.L.; Gardner, W.; Keller, N. Sporogenic Effect of Polyunsaturated Fatty Acids on Development of Aspergillus spp. Appl. Environ. Microbiol. 1999, 65, 3668–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, A.N.; Youssef, M.; Abdel-Razek, A.G.; Shehata, M.G.; Hassanien, M.M.; Amra, H. Natural antioxidants: Preservation roles and mycotoxicological safety of food. Egypt. J. Chem. 2021, 64, 285–298. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramirez, M.L. Evaluation of ability of ferulic acid to control growth and fumonisin production of Fusarium verticillioides and Fusarium proliferatum on maize based media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.M.; Wandiga, S.O.; Kariuki, D.K.; Madadi, V.O. Degradation of aflatoxin in maize using Ferulic acid (phydroxy-3-methyl cinnamic acid) catalyzed by Hydrogen peroxide. J. Food Sci. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [Green Version]
- Amin, T.; Naik, H.R.; Hussain, S.Z.; Jabeen, A.; Thakur, M. In-vitro antioxidant and antibacterial activities of pumpkin, quince, muskmelon and bottle gourd seeds. J. Food Meas. Charact. 2018, 12, 182–190. [Google Scholar] [CrossRef]
- Essien, E.; Antia, B.; Udoh, B.I. Phytochemical screening and antimicrobial activity of lagenaria siceraria seeds extracts. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 554–558. [Google Scholar]
- Bhat, S.; Saini, C.S.; Sharma, H.K. Changes in total phenolic content and color of bottle gourd (Lagenaria siceraria) juice upon conventional and ohmic blanching. Food Sci. Biotechnol. 2017, 26, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Abdel-Fattah, S.M.; Badr, A.N.; Ali, S.M.; Hassan, R.A. Antifungal and anti-mycotoxigenic impact of eco-friendly extracts of wild stevia. J. Biol. Sci. 2018, 18, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Razek, A.G.; Badr, A.N.; Shehata, M.G. Characterization of olive oil by-products: Antioxidant activity, its ability to reduce aflatoxigenic fungi hazard and its aflatoxins. Annu. Res. Rev. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, C.; Machado, A.; Cadoná, F.; Jaques, J.; Schlemmer, K.; Lautert, C.; Cruz, I.; Zanette, R.; Leal, D.; Santurio, J. In-vitro cytotoxicity of aflatoxin B1 to broiler lymphocytes of broiler chickens. Braz. J. Poult. Sci. 2014, 16, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, F.; Yazdanpanah, H.; Khoshjoo, F.; Hosseinzadeh, L. Pistachio Extracts Effects on the Aflatoxin B1 Cytotoxicity in HepG2 Cells. Int. J. Pharmacol. 2006, 2, 233–239. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Balz, M.K.; Schulte, E.; Thier, H.-P. Simultaneous Determination of α-Tocopheryl Acetate, Tocopherols and Tocotrienols by HPLC with Fluorescence Detection in Foods. Lipid 1993, 95, 215–220. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 2017, 33, 949–962. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compounds in Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.-C.; Duh, P.-D. Antioxidant activity of methanolic extracts of peanut hulls from various cultivars. J. Am. Oil Chem. Soc. 1995, 72, 1065. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Meneely, J.P.; Hajšlová, J.; Krska, R.; Elliott, C.T. Assessing the combined toxicity of the natural toxins, aflatoxin B1, fumonisin B1 and microcystin-LR by high content analysis. Food Chem. Toxicol. 2018, 121, 527–540. [Google Scholar] [CrossRef]
- Badr, A.N.; Logrieco, A.F.; Amra, H.A.; Hussein, T. Ochratoxin a occurrence on Egyptian wheat during seasons (2009–2014). Asian J. Sci. Res. 2017, 10, 178–185. [Google Scholar] [CrossRef] [Green Version]





| Material | Moisture | Fat | Total Protein | Total Carbohydrates | Ash | Crude Fiber |
|---|---|---|---|---|---|---|
| Seed powder | 4.92 ± 0.93 a | 34.12 ± 1.02 a | 11.54 ± 1.05 a | 19.21 ± 1.14 a | 2.51 ± 0.73 a | 32.41 ± 1.54 a |
| de-fatted powder | 6.37 ± 0.57 b | 2.17 ± 0.34 b | 18.61 ± 1.27 b | 31.18 ± 1.31 b | 4.27 ± 0.88 b | 52.11 ± 1.74 b |
| Fatty Acid | Concentration (%) | Tocols Compounds | µg/g |
|---|---|---|---|
| C16:0 (palmitic acid) | 6.41 ± 0.41 | α-tocopherol | 367.18 ± 4.26 |
| C16:1 (palmitoleic acid) | 0.39 ± 0.61 | β-tocopherol | ND |
| C17:0 (heptadecanoic acid) | ND | γ-tocopherol | 64.09 ± 2.14 |
| C17:1 (heptadecenoic acid) | ND | δ-tocopherol | 117.85 ± 3.71 |
| C18:0 (stearic acid) | 0.38 ± 0.18 | Total tocopherol | 549.12 ± 10.11 |
| C18:1 (oleic acid) | 49.37 ± 1.14 | µg/g | |
| C18:2n6 (linoleic acid) | 35.21 ± 1.05 | α-tocotrienol | 27.37 ± 1.67 |
| C18:3n6 (y-linolenic acid) | 7.51 ± 0.54 | β-tocotrienol | 1.25 ± 0.34 |
| C18:3n3 (linolenic acid) | 0.22 ± 0.08 | γ-tocotrienol | 45.61 ± 1.46 |
| C20:0 (arachidic acid) | 0.10 ± 0.02 | δ-tocotrienol | 18.12 ± 1.02 |
| C20:1 (c-11-eicosenoic acid) | ND | Total tocotrienol | 92.35 ± 4.49 |
| C20:2 (eicosadienoic acid) | ND | ||
| C21:0 (heneicosanoic acid) | ND | Sterols | mg/100 g |
| C22:1 (erukowy) | 0.18 ± 0.01 | Campesterol | 59.61 ± 2.05 |
| C24:0 (tetrakozanowy) | 0.20 ± 0.02 | Stigmasterol | 6.74 ± 0.54 |
| C24:1 (nerwonowy) | ND | Ergosterol | 22.40 ± 1.46 |
| β-sitosterol | 332.66 ± 5.71 | ||
| Oil-Significant values | δ-5-avenasterol | 1.94 ± 0.22 | |
| SFA | 7.09 | ||
| MUFA | 50.51 | Carotenoids | µg/g |
| PUFA | 42.94 | lutein | 109.78 ± 2.66 |
| SFA:MUFA:PUFA | 0.21:1.51:1.28 | Zeaxanthin | 294.24 ± 3.08 |
| Cox value of seeds oil | 5.8 | β-carotene | 674.16 ± 5.74 |
| Phenolic Acids | Concentrations in Polar Extract (µg/g) | Flavonoids | Concentrations in Polar Extract (µg/g) |
|---|---|---|---|
| Chlorogenic | 97.15 ± 1.58 | Apigenin | 105.3 ± 2.54 |
| Syringic | 6.25 ± 0.51 | Catechin | 45.2 ± 1.46 |
| 4-hydroxybenzoic | 71.6 ± 1.05 | Epicatechin | ND |
| Caffeic | 5.22 ± 0.41 | Luteolin | 0.19 ± 0.05 |
| Ferulic | 105.2 ± 2.88 | Rutin | ND |
| Gallic | 14.3 ± 0.97 | Naringin | 0.28 ± 0.06 |
| p-Cumaric | 52.1 ± 1.81 | Quercetin | ND |
| Protocatechuic | 0.3 ± 0.02 | Apigenin-7-glucoside | 0.31 ± 0.14 |
| Sinapic | 91.2 ± 1.37 | Kaempferol | 6.25 ± 0.51 |
| Vanilic | 0.56 ± 0.22 | Chrysin | 0.21 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Razek, A.G.; Badr, A.N.; Alharthi, S.S.; Selim, K.A. Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites. Toxins 2021, 13, 789. https://doi.org/10.3390/toxins13110789
Abdel-Razek AG, Badr AN, Alharthi SS, Selim KA. Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites. Toxins. 2021; 13(11):789. https://doi.org/10.3390/toxins13110789
Chicago/Turabian StyleAbdel-Razek, Adel G., Ahmed N. Badr, Salman S. Alharthi, and Khaled A. Selim. 2021. "Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites" Toxins 13, no. 11: 789. https://doi.org/10.3390/toxins13110789
APA StyleAbdel-Razek, A. G., Badr, A. N., Alharthi, S. S., & Selim, K. A. (2021). Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites. Toxins, 13(11), 789. https://doi.org/10.3390/toxins13110789